
Organic Chemistry
9th Edition
ISBN: 9781305080485
Author: John E. McMurry
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Chapter 4.SE, Problem 51AP
Interpretation Introduction
Interpretation:
The magnitude of energy difference is to be calculated considering the biaxial interaction.
Concept Introduction:
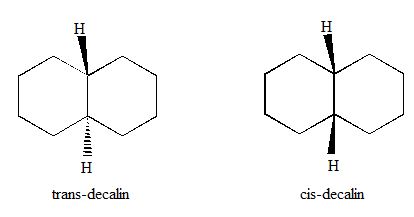
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
What is the total energy cost associated with the compound below adopting the shown conformation?
CH3
HH
DH
CH3
ΗΝ,
Draw Final Product
C
cyclohexanone
pH 4-5
Edit Enamine
H3O+
CH3CH2Br
THF, reflux
H
Edit Iminium Ion
How many hydrogen atoms are connected to the indicated carbon atom?
Chapter 4 Solutions
Organic Chemistry
Ch. 4.1 - Give IUPAC names for the following cycloalkanes:Ch. 4.1 - Draw structures corresponding to the following...Ch. 4.1 - Name the following cycloalkane:Ch. 4.2 - Prob. 4PCh. 4.2 - Draw the structures of the following molecules:...Ch. 4.2 - Prostaglandin F2α, a hormone that causes uterine...Ch. 4.2 - Name the following substances, including the cis-...Ch. 4.3 - Each H↔H eclipsing interaction in ethane costs...Ch. 4.3 - cis-1, 2-Dimethylcyclopropane has more strain than...Ch. 4.4 - Prob. 10P
Ch. 4.4 - Two conformations of cis-l, 3-dimethylcyclobutane...Ch. 4.6 - Draw two different chair conformations of...Ch. 4.6 - Draw two differant chair conformations of trans-1,...Ch. 4.6 - Prob. 14PCh. 4.7 - What is the energy difference between the axial...Ch. 4.7 - Prob. 16PCh. 4.7 - Look at Figure 4-12 on page 105, and estimate the...Ch. 4.8 - Draw the more stable chair conformation of the...Ch. 4.8 - Identify each substituent in the following...Ch. 4.9 - Which isomer is more stable, cis-decalin or...Ch. 4.9 - Look at the following structure of the female...Ch. 4.SE - Prob. 22VCCh. 4.SE - Name the following compound, identify each...Ch. 4.SE - A trisubstituted cyclohexane with three...Ch. 4.SE - The following cyclohexane derivative has three...Ch. 4.SE - Prob. 26VCCh. 4.SE - Draw the five cycloalkanes with the formula C5H10.Ch. 4.SE - Draw two constitutional isomers of cis-1,...Ch. 4.SE - Prob. 29APCh. 4.SE - Tell whether the following pairs of compounds are...Ch. 4.SE - Prob. 31APCh. 4.SE - Prob. 32APCh. 4.SE - Draw 1, 3, 5-trimethylcyclohexane using a hexagon...Ch. 4.SE - Hydrocortisone, a naturally occurring hormone...Ch. 4.SE - A 1, 2-cis disubstituted cyclohexane, such as...Ch. 4.SE - A 1, 2-trans disubstituted cyclohexane must have...Ch. 4.SE - Prob. 37APCh. 4.SE - Which is more stable, a 1, 4-trans disubstituted...Ch. 4.SE - cis-1, 2-Dimethylcyclobutane is less stable than...Ch. 4.SE - From the data in Figure 4-12 and Table 4-1,...Ch. 4.SE - Prob. 41APCh. 4.SE - Draw the two chair conformations of...Ch. 4.SE - Draw the two chair conformations of...Ch. 4.SE - Galactose, a sugar related to glucose, contains a...Ch. 4.SE - There are four cis-trans isomers of menthol...Ch. 4.SE - There are four cis-trans isomers of menthol...Ch. 4.SE - The diaxial conformation of cis-1,...Ch. 4.SE - Approximately how much steric strain does the...Ch. 4.SE - In light of your answer to Problem 4-43, draw the...Ch. 4.SE - Prob. 50APCh. 4.SE - Prob. 51APCh. 4.SE - Using molecular models as well as structural...Ch. 4.SE - trans-Decalin is more stable than its cis isomer,...Ch. 4.SE - As mentioned in Problem 3-53, the statin drugs,...Ch. 4.SE - myo-Inositol, one of the isomers of...Ch. 4.SE - How many cis–trans stereoisomers of myo-inositol...Ch. 4.SE - The German chemist J. Bredt proposed in 1935 that...Ch. 4.SE - Tell whether each of the following substituents on...Ch. 4.SE - Prob. 59APCh. 4.SE - Prob. 60APCh. 4.SE - Ketones react with alcohols to yield products...Ch. 4.SE - Alcohols undergo an oxidation reaction to yield...
Knowledge Booster
Similar questions
- Identify the compound with the longest carbon - nitrogen bond. O CH3CH2CH=NH O CH3CH2NH2 CH3CH2C=N CH3CH=NCH 3 The length of all the carbon-nitrogen bonds are the samearrow_forwardIdentify any polar covalent bonds in epichlorohydrin with S+ and 8- symbols in the appropriate locations. Choose the correct answer below. Η H's+ 6Η Η Η Η Η Ηδ Η Ο Ο HH +Η Η +Η Η Η -8+ CIarrow_forwardH H:O::::H H H HH H::O:D:D:H HH HH H:O:D:D:H .. HH H:O:D:D:H H H Select the correct Lewis dot structure for the following compound: CH3CH2OHarrow_forward
- Rank the following compounds in order of decreasing boiling point. ннннн -С-С-Н . н-с- ННННН H ΗΤΗ НННН TTTĪ н-с-с-с-с-о-н НННН НН C' Н н-с-с-с-с-н НН || Ш НННН H-C-C-C-C-N-H ННННН IVarrow_forwardRank the following compounds in order of decreasing dipole moment. |>||>||| ||>|||>| |>|||>|| |||>||>| O ||>>||| H F H F H c=c || H c=c F F IIIarrow_forwardchoose the description that best describes the geometry for the following charged species ch3-arrow_forward
- Why isn't the ketone in this compound converted to an acetal or hemiacetal by the alcohol and acid?arrow_forwardWhat is the approximate bond angle around the nitrogen atom? HNH H Harrow_forwardOH 1. NaOCH2CH3 Q 2. CH3CH2Br (1 equiv) H3O+ Select to Draw 1. NaOCH2 CH3 2. CH3Br (1 equiv) heat Select to Edit Select to Drawarrow_forward
- Complete and balance the following half-reaction in acidic solution. Be sure to include the proper phases for all species within the reaction. S₂O₃²⁻(aq) → S₄O₆²⁻(aq)arrow_forwardQ Select to Edit NH3 (CH3)2CHCI (1 equiv) AICI 3 Select to Draw cat. H2SO4 SO3 (1 equiv) HO SOCl2 pyridine Select to Edit >arrow_forwardComplete and balance the following half-reaction in basic solution. Be sure to include the proper phases for all species within the reaction. Zn(s) → Zn(OH)₄²⁻(aq)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning


Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning