
Introduction To General, Organic, And Biochemistry
12th Edition
ISBN: 9781337571357
Author: Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 4.6, Problem 4.10QC
Problem 4-10
How many moles of copper(I) ions, Cu+, are there in 0.062 g of copper(I) nitrate, 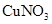
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Part II. Given two isomers: 2-methylpentane (A) and 2,2-dimethyl butane (B) answer the following:
(a) match structures of isomers given their mass spectra below (spectra A and spectra B)
(b) Draw the fragments given the following prominent peaks from
each spectrum:
Spectra A m/2 =43 and 1/2-57
spectra B m/2 = 43
(c) why is 1/2=57 peak in spectrum A more intense compared
to the same peak in spectrum B.
Relative abundance
Relative abundance
100
A
50
29
29
0
10
-0
-0
100
B
50
720
30
41
43
57
71
4-0
40
50
60 70
m/z
43
57
8-0
m/z = 86
M
90 100
71
m/z = 86
M
-O
0
10 20 30
40 50
60
70
80
-88
m/z
90
100
Part IV. C6H5 CH2CH2OH is an aromatic compound which was subjected to Electron Ionization - mass
spectrometry (El-MS) analysis. Prominent m/2 values: m/2 = 104 and m/2 = 9) was obtained.
Draw the structures of these fragments.
For each reaction shown below follow the curved arrows to complete each equationby showing the structure of the products. Identify the acid, the base, the conjugated acid andconjugated base. Consutl the pKa table and choose the direciton theequilibrium goes. However show the curved arrows. Please explain if possible.
Chapter 4 Solutions
Introduction To General, Organic, And Biochemistry
Ch. 4.2 - Problem 4-1 Following is an unbalanced equation...Ch. 4.2 - Problem 4-2 Balance this equation:Ch. 4.2 - Prob. 4.3QCCh. 4.3 - Problem 4-4 When a solution of copper(II)...Ch. 4.4 - Problem 4-5 In each equation, identify the...Ch. 4.5 - Problem 4-6 What is (a) the molecular weight of...Ch. 4.6 - Prob. 4.7QCCh. 4.6 - Problem 4-8 We wish to weigh 2.84 mol of sodium...Ch. 4.6 - Problem 4-9 How many moles of C atoms, H atoms,...Ch. 4.6 - Problem 4-10 How many moles of copper(I) ions,...
Ch. 4.6 - Prob. 4.11QCCh. 4.7 - Prob. 4.12QCCh. 4.7 - Prob. 4.13QCCh. 4.7 - Problem 4-14 Ethanol is produced industrially by...Ch. 4.7 - Prob. 4.15QCCh. 4.7 - Prob. 4.16QCCh. 4.8 - How many calories are required to heat 731g of...Ch. 4.8 - A 100g piece of iron at 25C is heated by adding...Ch. 4.8 - It required 88.2 cal to heat 13.4g of an unknown...Ch. 4.9 - Solid iron and oxygen gas react to form solid...Ch. 4 - 4-17 Balance each equation.Ch. 4 - 4-18 Balance each equation.Ch. 4 - Prob. 3PCh. 4 - 4-20 Calcium oxide is prepared by heating...Ch. 4 - 4-21 The brilliant white light in some firework...Ch. 4 - Prob. 6PCh. 4 - 4-23 When solid carbon burns in a limited supply...Ch. 4 - Prob. 8PCh. 4 - 4-25 In the chemical test for arsenic, the gas...Ch. 4 - Prob. 10PCh. 4 - Prob. 11PCh. 4 - 4-28 Answer true or false. (a) A net ionic...Ch. 4 - 4-29 Balance these net ionic equations. (a)...Ch. 4 - 4-30 In the equation (a) Identify the spectator...Ch. 4 - 4-31 Predict whether a precipitate will form when...Ch. 4 - 4-32 When a solution of ammonium chloride is added...Ch. 4 - 4-33 When a solution of hydrochloric acid, HCl, is...Ch. 4 - Prob. 18PCh. 4 - Prob. 19PCh. 4 - 4-36 Using the solubility generalizations given in...Ch. 4 - 4-37 Answer true or false. (a) When a substance is...Ch. 4 - Prob. 22PCh. 4 - Prob. 23PCh. 4 - Prob. 24PCh. 4 - Prob. 25PCh. 4 - 4-42 Calculate the formula weight of: (a) KCl (b)...Ch. 4 - 4-43 Calculate the molecular weight of: (a)...Ch. 4 - 4-44 Answer true or false. (a) The mole is a...Ch. 4 - 4-45 Calculate the number of moles in: (a) 32 g of...Ch. 4 - 4-46 Calculate the number of grams in: (a) 1.77...Ch. 4 - 4-47 Calculate the number of moles of: (a) O atoms...Ch. 4 - 4-48 Calculate the number of moles of: (a) S2-...Ch. 4 - 4-49 Calculate the number of: (a) nitrogen atoms...Ch. 4 - 4-50 How many molecules are in each of the...Ch. 4 - 4-51 What is the mass in grams of each number of...Ch. 4 - 4-52 The molecular weight of hemoglobin is about...Ch. 4 - 4-53 A typical deposit of cholesterol, C27H46O, in...Ch. 4 - 4-54 Answer true or false. (a) Stoichiometry is...Ch. 4 - 4-55 For the reaction: (a) How many moles of N2...Ch. 4 - 4-56 Magnesium reacts with sulfuric acid according...Ch. 4 - 4-57 Chloroform, CHCl3, is prepared industrially...Ch. 4 - 4-58 At one time, acetaldehyde was prepared...Ch. 4 - 4-59 Chlorine dioxide, ClO2, is used for bleaching...Ch. 4 - 4-60 Ethanol, C2H6O, is added to gasoline to...Ch. 4 - 4-61 In photosynthesis, green plants convert CO2...Ch. 4 - 4-62 Iron ore is converted to iron by heating it...Ch. 4 - Prob. 47PCh. 4 - 4-64 Aspirin is made by the reaction of salicylic...Ch. 4 - 4-65 Suppose the preparation of aspirin from...Ch. 4 - 4-66 Benzene reacts with bromine to produce...Ch. 4 - 4-67 Ethyl chloride is prepared by the reaction of...Ch. 4 - 4-68 Diethyl ether is made from ethanol according...Ch. 4 - Prob. 53PCh. 4 - How many calories are required to heat the...Ch. 4 - Prob. 55PCh. 4 - Prob. 56PCh. 4 - Prob. 57PCh. 4 - Prob. 58PCh. 4 - 4-71 Which of these reactions are exothermic, and...Ch. 4 - Prob. 60PCh. 4 - Prob. 61PCh. 4 - Prob. 62PCh. 4 - Prob. 63PCh. 4 - Prob. 64PCh. 4 - 4-77 To convert 1 mol of iron(III) oxide to its...Ch. 4 - 4-78 (Chemical Connections 4A) How does fluoride...Ch. 4 - Prob. 67PCh. 4 - Prob. 68PCh. 4 - 4-81 (Chemical Connections 4C) Balance the lithium...Ch. 4 - 4-82 When gaseous dinitrogen pentoxide, N2O5, is...Ch. 4 - Prob. 71PCh. 4 - Prob. 72PCh. 4 - Prob. 73PCh. 4 - 4-86 When an aqueous solution of Na3PO4 is added...Ch. 4 - Prob. 75PCh. 4 - 4-88 Chlorophyll, the compound responsible for the...Ch. 4 - 4-89 If 7.0 kg of is added to 11.0 kg of to form...Ch. 4 - 4-90 Lead(lI) nitrate and aluminum chloride react...Ch. 4 - 4-91 Assume that the average red blood cell has a...Ch. 4 - 4-92 Reaction of pentane, C5H12, with oxygen, O2,...Ch. 4 - 4-93 Ammonia is prepared industrially by the...Ch. 4 - 4-94 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) is...Ch. 4 - Prob. 83PCh. 4 - Prob. 84PCh. 4 - Prob. 85PCh. 4 - Prob. 86PCh. 4 - Prob. 87PCh. 4 - Prob. 88PCh. 4 - Prob. 89PCh. 4 - Prob. 90PCh. 4 - Prob. 91PCh. 4 - 4-102 Aspartame, an artificial sweetener used as a...Ch. 4 - 4-103 Caffeine, a central nervous system...Ch. 4 - Prob. 94P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A molecule shows peaks at 1379, 1327, 1249, 739 cm-1. Draw a diagram of the energy levels for such a molecule. Draw arrows for the possible transitions that could occur for the molecule. In the diagram imagine exciting an electron, what are its various options for getting back to the ground state? What process would promote radiation less decay? What do you expect for the lifetime of an electron in the T1 state? Why is phosphorescence emission weak in most substances? What could you do to a sample to enhance the likelihood that phosphorescence would occur over radiationless decay?arrow_forwardRank the indicated C—C bonds in increasing order of bond length. Explain as why to the difference.arrow_forwardUse IUPAC rules to name the following alkanearrow_forward
- Please correct answer and don't use hand ratingarrow_forwardPlease correct answer and don't use hand ratingarrow_forwardThe SN 1 mechanism starts with the rate-determining step which is the dissociation of the alkyl halide into a carbocation and a halide ion. The next step is the rapid reaction of the carbocation intermediate with the nucleophile; this step completes the nucleophilic substitution stage. The step that follows the nucleophilic substitution is a fast acid-base reaction. The nucleophile now acts as a base to remove the proton from the oxonium ion from the previous step, to give the observed product. Draw a curved arrow mechanism for the reaction, adding steps as necessary. Be sure to include all nonzero formal charges. Cl: Add/Remove step G Click and drag to start drawing a structure.arrow_forward
- Please correct answer and don't use hand ratingarrow_forwardA monochromatic light with a wavelength of 2.5x10-7m strikes a grating containing 10,000 slits/cm. Determine the angular positions of the second-order bright line.arrow_forwardCurved arrows are used to illustrate the flow of electrons. Us the reaction conditions provided and follow the curved arrow to draw the resulting structure(s). Include all lone pairs and charges as appropriate. H :I H 0arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning
Bonding (Ionic, Covalent & Metallic) - GCSE Chemistry; Author: Science Shorts;https://www.youtube.com/watch?v=p9MA6Od-zBA;License: Standard YouTube License, CC-BY
Stoichiometry - Chemistry for Massive Creatures: Crash Course Chemistry #6; Author: Crash Course;https://www.youtube.com/watch?v=UL1jmJaUkaQ;License: Standard YouTube License, CC-BY