
4-31 Predict whether a precipitate will form when aqueous solutions of the following compounds are mixed. If a precipitate will form, write its formula and write a net ionic equation for its formation. To make your predictions, use the solubiity generalizations in
Section 4-3.
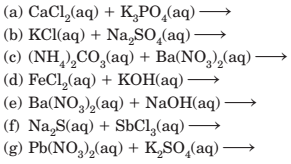
(a)
Interpretation:
Whether the given reaction gives precipitate or not should be determined. If yes, the formula of the product should be written along with the net ionic equation.
Concept Introduction:
When two aqueous solutions are mixed together, this may or may not result in formation of an insoluble solid in the solution. This insoluble solid is called precipitate and this reaction is called precipitation reaction.
For example, a reaction in which A and C displace each other from their respective solutions. Since two displacement takes place, this reaction is also called double displacement reaction.
Here,
Answer to Problem 15P
Phosphate of calcium are insoluble, hence they separate out as precipitate.
Precipitate -
Net Reaction.
Explanation of Solution
The given reaction is as-
If the double displacement-reaction takes place ,then the product of the reaction would be-
The products are phosphate of calcium and chloride of potassium.
Phosphates of calcium are insoluble; hence, they separate out as precipitate, chlorides of potassium are soluble, they remain as aqueous solution.
The net reaction is as-
(b)
Interpretation:
The precipitation or double-displacement reaction is to be determined.
Concept Introduction:
When two aqueous solutions are mixed together, this may or may not result in formation of an insoluble solid in the solution. This insoluble solid is called precipitate and this reaction is called precipitation reaction.
For example, a reaction in which A and C displace each other from their respective solutions. Since two displacement takes place, this reaction is also called double displacement reaction.
Here, AD(s) represents a precipitate.
Answer to Problem 15P
No precipitate.
Net Reaction.
Explanation of Solution
The given reaction is as-
If the double displacement-reaction takes place ,then the product of the reaction would be-
The products are sulphate of potassium and chloride of Sodium.
Sulphates of potassium are soluble, they remain as aqueous solution.
Chlorides of sodium are soluble, they remain as aqueous solution.
The net reaction is as-
(c)
Interpretation:
The precipitation or double-displacement reaction is to be determined.
Concept Introduction:
When two aqueous solutions are mixed together, this may or may not result in formation of an insoluble solid in the solution. This insoluble solid is called precipitate and this reaction is called precipitation reaction.
For example, a reaction in which A and C displace each other from their respective solutions. Since two displacement takes place, this reaction is also called double displacement reaction.
Here, AD(s) represents a precipitate.
Answer to Problem 15P
Phosphate of Calcium are insoluble, hence they separate out as precipitate.
Precipitate -
Net Reaction.
Explanation of Solution
The given reaction is as-
If the double displacement-reaction takes place ,then the product of the reaction would be-
The products are Carbonate of Barium and Nitrate of Ammonium.
Carbonate of Barium are insoluble, hence they separate out as precipitate.
Nitrate of Ammonium are soluble, they remain as aqueous solution.
The net reaction is as-
(d)
Interpretation:
The precipitation or double-displacement reaction is to be determined.
Concept Introduction:
When two aqueous solutions are mixed together, this may or may not result in formation of an insoluble solid in the solution. This insoluble solid is called precipitate and this reaction is called precipitation reaction.
For example, a reaction in which A and C displace each other from their respective solutions. Since two displacement takes place, this reaction is also called double displacement reaction.
Here, AD(s) represents a precipitate.
Answer to Problem 15P
No precipitate.
Net Reaction.
Explanation of Solution
The given reaction is as-
If the double displacement-reaction takes place, then the product of the reaction would be-
The products are Chloride of Potassium and Hydroxide of Iron.
Chloride of Potassium are soluble, they remain as aqueous solution.
Hydroxide of Iron are soluble, they remain as aqueous solution.
The net reaction is as-
(e)
Interpretation:
The precipitation or double-displacement reaction is to be determined.
Concept Introduction:
When two aqueous solutions are mixed together, this may or may not result in formation of an insoluble solid in the solution. This insoluble solid is called precipitate and this reaction is called precipitation reaction.
For example, a reaction in which A and C displace each other from their respective solutions. Since two displacement takes place, this reaction is also called double displacement reaction.
Here, AD(s) represents a precipitate.
Answer to Problem 15P
No precipitate.
Net Reaction.
Explanation of Solution
The given reaction is as-
If the double displacement-reaction takes place, then the product of the reaction would be-
The products are Hydroxide of Barium and Nitrate of Sodium.
Hydroxide of Barium are soluble, they remain as aqueous solution.
Nitrate of Sodium are soluble, they remain as aqueous solution.
The net reaction is as-
(f)
Interpretation:
The precipitation or double-displacement reaction is to be determined.
Concept Introduction:
When two aqueous solutions are mixed together, this may or may not result in formation of an insoluble solid in the solution. This insoluble solid is called precipitate and this reaction is called precipitation reaction.
For example, a reaction in which A and C displace each other from their respective solutions. Since two displacement takes place, this reaction is also called double displacement reaction.
Here, AD(s) represents a precipitate.
Answer to Problem 15P
Phosphate of Calcium are insoluble, hence they separate out as precipitate.
Precipitate -
Net Reaction.
Explanation of Solution
The given reaction is as-
If the double displacement-reaction takes place, then the product of the reaction would be-
The products are Chloride of Sodium and Sulphide of Antimony.
Chloride of Sodium are soluble, they remain as aqueous solution.
Sulphide of Antimony are insoluble, hence they separate out as precipitate.
The net reaction is as-
(g)
Interpretation:
The precipitation or double-displacement reaction is to be determined.
Concept Introduction:
When two aqueous solutions are mixed together, this may or may not result in formation of an insoluble solid in the solution. This insoluble solid is called precipitate and this reaction is called precipitation reaction.
For example, a reaction in which A and C displace each other from their respective solutions. Since two displacement takes place, this reaction is also called double displacement reaction.
Here, AD(s) represents a precipitate.
Answer to Problem 15P
Sulphate of Lead are insoluble, hence they separate out as precipitate.
Precipitate -
Net Reaction.
Explanation of Solution
The given reaction is as-
If the double displacement-reaction takes place, then the product of the reaction would be-
The products are Sulphate of Lead and Nitrate of Potassium.
Chloride of Sodium are soluble, they remain as aqueous solution.
Sulphate of Lead are insoluble, hence they separate out as precipitate.
The net reaction is as-
Want to see more full solutions like this?
Chapter 4 Solutions
Introduction To General, Organic, And Biochemistry
- ASP please....arrow_forwardNonearrow_forwardConsider the structure of 1-bromo-2-fluoroethane. Part 1 of 2 Draw the Newman projection for the anti conformation of 1-bromo-2-fluoroethane, viewed down the C1-C2 bond. ✡ ぬ Part 2 of 2 H H F Br H H ☑ Draw the Newman projection for the gauche conformation of 1-bromo-2-fluoroethane, viewed down the C1-C2 bond. H F Br H Harrow_forward
- Please help me answer this question. I don't understand how or where the different reagents will attach and it's mostly due to the wedge bond because I haven't seen a problem like this before. Please provide a detailed explanation and a drawing showing how it can happen and what the final product will look like.arrow_forwardWhich of the following compounds is the most acidic in the gas phase? Group of answer choices H2O SiH4 HBr H2Sarrow_forwardWhich of the following is the most acidic transition metal cation? Group of answer choices Fe3+ Sc3+ Mn4+ Zn2+arrow_forward
- Based on the thermodynamics of acetic acid dissociation discussed in Lecture 2-5, what can you conclude about the standard enthalpy change (ΔHo) of acid dissociation for HCl? Group of answer choices You cannot arrive at any of the other three conclusions It is a positive value It is more negative than −0.4 kJ/mol It equals −0.4 kJ/molarrow_forwardPLEASE HELP URGENT!arrow_forwardDraw the skeletal structure corresponding to the following IUPAC name: 7-isopropyl-3-methyldecanearrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning





