
Concept explainers
(a)
Interpretation:
Enatiomers for the given compound has to be drawn using perspective formula.
Concept Introduction:
Perspective formulas show the 3D array of atoms in which the solid wedges indicate bonds projecting above the plane of the drawing and wedge shaded with parallel line indicate bonds projecting below the plane of drawing.
Example:
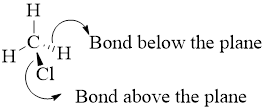
Enantiomers: These are stereoisomers that are not superimposable mirror images of each other and the configurations at all stereo genic centers are exactly opposite.
Enantiomer can be drawn by replacing the wedge with a dash from the perspective formula of a compound.
Chiral center: A chiral center is defined as the tetrahedral carbon atom in an organic molecule that is connected to four non-identical groups/substituents. It is sometimes known as a stereo genic center.
An achiral carbon is a carbon having two or more identical groups around it.
(b)
Interpretation:
Enatiomers for the given compound has to be drawn using perspective formula.
Concept Introduction:
Perspective formulas show the 3D array of atoms in which the solid wedges indicate bonds projecting above the plane of the drawing nad wedge shaded with parallel line indicate bonds projecting below the plane of drawing.
Example:
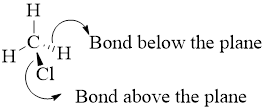
Enantiomers: These are stereoisomers that are not superimposable mirror images of each other and the configurations at all stereo genic centers are exactly opposite.
Enantiomer can be drawn by replacing the wedge with a dash from the perspective formula of a compound.
Chiral center: A chiral center is defined as the tetrahedral carbon atom in an organic molecule that is connected to four non-identical groups/substituents. It is sometimes known as a stereo genic center.
An achiral carbon is a carbon having two or more identical groups around it.
(c)
Interpretation:
Enatiomers for the given compound has to be drawn using perspective formula.
Concept Introduction:
Perspective formulas show the 3D array of atoms in which the solid wedges indicate bonds projecting above the plane of the drawing nad wedge shaded with parallel line indicate bonds projecting below the plane of drawing.
Example:
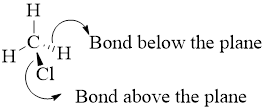
Enantiomers: These are stereoisomers that are not superimposable mirror images of each other and the configurations at all stereo genic centers are exactly opposite.
Enantiomer can be drawn by replacing the wedge with a dash from the perspective formula of a compound.
Chiral center: A chiral center is defined as the tetrahedral carbon atom in an organic molecule that is connected to four non-identical groups/substituents. It is sometimes known as a stereo genic center.
An achiral carbon is a carbon having two or more identical groups around it.
Want to see the full answer?
Check out a sample textbook solution
Chapter 4 Solutions
EBK ESSENTIAL ORGANIC CHEMISTRY
- The electrons flow from the electron-rich atoms of the nucleophile to the electrons poor atoms of the alkyl halide. Identify the electron rich in the nucleophile. Enter the element symbol only, do not include any changes.arrow_forwardHello, I am doing a court case analysis in my Analytical Chemistry course. The case is about a dog napping and my role is prosecution of the defendant. I am tasked in the Area of Expertise in Neutron Activation and Isotopic Analysis. Attached is the following case study reading of my area of expertise! The landscaping stone was not particularly distinctive in its decoration but matched both the color and pattern of the Fluential’s landscaping stone as well as the stone in the back of the recovered vehicle. Further analysis of the stone was done using a technique called instrumental neutron activation analysis. (Proceed to Neutron Activation data) Photo Notes: Landscaping stone recovered in vehicle. Stone at Fluential’s home is similar inappearance. Finally, the white paint on the brick was analyzed using stable isotope analysis. The brick recovered at the scene had smeared white paint on it. A couple of pieces of brick in the back of the car had white paint on them. They…arrow_forwardCite the stability criteria of an enamine..arrow_forward
- What would you expect to be the major product obtained from the following reaction? Please explain what is happening here. Provide a detailed explanation and a drawing showing how the reaction occurs. The correct answer to this question is V.arrow_forwardPlease answer the question for the reactions, thank youarrow_forwardWhat is the product of the following reaction? Please include a detailed explanation of what is happening in this question. Include a drawing showing how the reagent is reacting with the catalyst to produce the correct product. The correct answer is IV.arrow_forward
- Please complete the reactions, thank youarrow_forwardConsider the synthesis. What is compound Y? Please explain what is happening in this question. Provide a detailed explanation and a drawing to show how the compound Y creates the product. The correct answer is D.arrow_forwardWhat would be the major product of the following reaction? Please include a detailed explanation of what is happening in this question. Include steps and a drawing to show this reaction proceeds and how the final product is formed. The correct answer is B. I put answer D and I don't really understand what is going on in the question.arrow_forward

 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


