
Bundle: Introduction to General, Organic and Biochemistry, 11th + OWLv2, 4 terms (24 months) Printed Access Card
11th Edition
ISBN: 9781305705159
Author: Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 4.2, Problem 4.2P
Problem 4-2 Balance this equation:
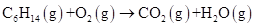
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Learning Goal:
This question reviews the format for writing an element's written symbol. Recall that written symbols have a particular format. Written symbols use a form like this:
35 Cl
17
In this form the mass number, 35, is a stacked superscript. The atomic number, 17, is a stacked subscript. "CI" is the chemical symbol for the element chlorine. A general way to show this form is:
It is also correct to write symbols by leaving off the atomic number, as in the following form:
atomic number
mass number Symbol
35 Cl or
mass number Symbol
This is because if you write the element symbol, such as Cl, you know the atomic number is 17 from that symbol. Remember that the atomic number, or number of protons in the nucleus, is what defines the element. Thus, if 17 protons
are in the nucleus, the element can only be chlorine. Sometimes you will only see 35 C1, where the atomic number is not written.
Watch this video to review the format for written symbols.
In the following table each column…
need help please and thanks dont understand only need help with C-F
Learning Goal:
As discussed during the lecture, the enzyme HIV-1 reverse transcriptae (HIV-RT) plays a significant role for the HIV virus and is an important drug target. Assume a concentration [E] of 2.00 µM (i.e. 2.00 x 10-6 mol/l) for HIV-RT. Two potential drug molecules, D1 and D2, were identified, which form stable complexes with the HIV-RT.
The dissociation constant of the complex ED1 formed by HIV-RT and the drug D1 is 1.00 nM (i.e. 1.00 x 10-9). The dissociation constant of the complex ED2 formed by HIV-RT and the drug D2 is 100 nM (i.e. 1.00 x 10-7).
Part A - Difference in binding free eenergies
Compute the difference in binding free energy (at a physiological temperature T=310 K) for the complexes. Provide the difference as a positive numerical expression with three significant figures in kJ/mol.
The margin of error is 2%.
Part B - Compare difference in free energy to the thermal…
need help please and thanks dont understand only need help with C-F
Learning Goal:
As discussed during the lecture, the enzyme HIV-1 reverse transcriptae (HIV-RT) plays a significant role for the HIV virus and is an important drug target. Assume a concentration [E] of 2.00 µM (i.e. 2.00 x 10-6 mol/l) for HIV-RT. Two potential drug molecules, D1 and D2, were identified, which form stable complexes with the HIV-RT.
The dissociation constant of the complex ED1 formed by HIV-RT and the drug D1 is 1.00 nM (i.e. 1.00 x 10-9). The dissociation constant of the complex ED2 formed by HIV-RT and the drug D2 is 100 nM (i.e. 1.00 x 10-7).
Part A - Difference in binding free eenergies
Compute the difference in binding free energy (at a physiological temperature T=310 K) for the complexes. Provide the difference as a positive numerical expression with three significant figures in kJ/mol.
The margin of error is 2%.
Part B - Compare difference in free energy to the thermal…
Chapter 4 Solutions
Bundle: Introduction to General, Organic and Biochemistry, 11th + OWLv2, 4 terms (24 months) Printed Access Card
Ch. 4.2 - Problem 4-1 Following is an unbalanced equation...Ch. 4.2 - Problem 4-2 Balance this equation:Ch. 4.2 - Prob. 4.3PCh. 4.3 - Problem 4-4 When a solution of copper(II)...Ch. 4.4 - Problem 4-5 In each equation, identify the...Ch. 4.5 - Problem 4-6 What is (a) the molecular weight of...Ch. 4.6 - Prob. 4.7PCh. 4.6 - Problem 4-8 We wish to weigh 2.84 mol of sodium...Ch. 4.6 - Problem 4-9 How many moles of C atoms, H atoms,...Ch. 4.6 - Problem 4-10 How many moles of copper(I) ions,...
Ch. 4.6 - Prob. 4.11PCh. 4.7 - Prob. 4.12PCh. 4.7 - Prob. 4.13PCh. 4.7 - Problem 4-14 Ethanol is produced industrially by...Ch. 4.7 - Prob. 4.15PCh. 4.7 - Prob. 4.16PCh. 4 - 4-17 Balance each equation.Ch. 4 - 4-18 Balance each equation.Ch. 4 - Prob. 4.19PCh. 4 - 4-20 Calcium oxide is prepared by heating...Ch. 4 - 4-21 The brilliant white light in some firework...Ch. 4 - Prob. 4.22PCh. 4 - 4-23 When solid carbon burns in a limited supply...Ch. 4 - Prob. 4.24PCh. 4 - 4-25 In the chemical test for arsenic, the gas...Ch. 4 - Prob. 4.26PCh. 4 - Prob. 4.27PCh. 4 - 4-28 Answer true or false. (a) A net ionic...Ch. 4 - 4-29 Balance these net ionic equations. (a)...Ch. 4 - 4-30 In the equation (a) Identify the spectator...Ch. 4 - 4-31 Predict whether a precipitate will form when...Ch. 4 - 4-32 When a solution of ammonium chloride is added...Ch. 4 - 4-33 When a solution of hydrochloric acid, HCl, is...Ch. 4 - Prob. 4.34PCh. 4 - Prob. 4.35PCh. 4 - 4-36 Using the solubility generalizations given in...Ch. 4 - 4-37 Answer true or false. (a) When a substance is...Ch. 4 - Prob. 4.38PCh. 4 - Prob. 4.39PCh. 4 - Prob. 4.40PCh. 4 - Prob. 4.41PCh. 4 - 4-42 Calculate the formula weight of: (a) KCl (b)...Ch. 4 - 4-43 Calculate the molecular weight of: (a)...Ch. 4 - 4-44 Answer true or false. (a) The mole is a...Ch. 4 - 4-45 Calculate the number of moles in: (a) 32 g of...Ch. 4 - 4-46 Calculate the number of grams in: (a) 1.77...Ch. 4 - 4-47 Calculate the number of moles of: (a) O atoms...Ch. 4 - 4-48 Calculate the number of moles of: (a) S2-...Ch. 4 - 4-49 Calculate the number of: (a) nitrogen atoms...Ch. 4 - 4-50 How many molecules are in each of the...Ch. 4 - 4-51 What is the mass in grams of each number of...Ch. 4 - 4-52 The molecular weight of hemoglobin is about...Ch. 4 - 4-53 A typical deposit of cholesterol, C27H46O, in...Ch. 4 - 4-54 Answer true or false. (a) Stoichiometry is...Ch. 4 - 4-55 For the reaction: (a) How many moles of N2...Ch. 4 - 4-56 Magnesium reacts with sulfuric acid according...Ch. 4 - 4-57 Chloroform, CHCl3, is prepared industrially...Ch. 4 - 4-58 At one time, acetaldehyde was prepared...Ch. 4 - 4-59 Chlorine dioxide, ClO2, is used for bleaching...Ch. 4 - 4-60 Ethanol, C2H6O, is added to gasoline to...Ch. 4 - 4-61 In photosynthesis, green plants convert CO2...Ch. 4 - 4-62 Iron ore is converted to iron by heating it...Ch. 4 - Prob. 4.63PCh. 4 - 4-64 Aspirin is made by the reaction of salicylic...Ch. 4 - 4-65 Suppose the preparation of aspirin from...Ch. 4 - 4-66 Benzene reacts with bromine to produce...Ch. 4 - 4-67 Ethyl chloride is prepared by the reaction of...Ch. 4 - 4-68 Diethyl ether is made from ethanol according...Ch. 4 - Prob. 4.69PCh. 4 - Prob. 4.70PCh. 4 - 4-71 Which of these reactions are exothermic, and...Ch. 4 - Prob. 4.72PCh. 4 - Prob. 4.73PCh. 4 - Prob. 4.74PCh. 4 - Prob. 4.75PCh. 4 - Prob. 4.76PCh. 4 - 4-77 To convert 1 mol of iron(III) oxide to its...Ch. 4 - 4-78 (Chemical Connections 4A) How does fluoride...Ch. 4 - Prob. 4.79PCh. 4 - Prob. 4.80PCh. 4 - 4-81 (Chemical Connections 4C) Balance the lithium...Ch. 4 - 4-82 When gaseous dinitrogen pentoxide, N2O5, is...Ch. 4 - Prob. 4.83PCh. 4 - Prob. 4.84PCh. 4 - Prob. 4.85PCh. 4 - 4-86 When an aqueous solution of Na3PO4 is added...Ch. 4 - Prob. 4.87PCh. 4 - 4-88 Chlorophyll, the compound responsible for the...Ch. 4 - 4-89 If 7.0 kg of is added to 11.0 kg of to form...Ch. 4 - 4-90 Lead(lI) nitrate and aluminum chloride react...Ch. 4 - 4-91 Assume that the average red blood cell has a...Ch. 4 - 4-92 Reaction of pentane, C5H12, with oxygen, O2,...Ch. 4 - 4-93 Ammonia is prepared industrially by the...Ch. 4 - 4-94 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) is...Ch. 4 - Prob. 4.95PCh. 4 - Prob. 4.96PCh. 4 - Prob. 4.97PCh. 4 - Prob. 4.98PCh. 4 - Prob. 4.99PCh. 4 - Prob. 4.100PCh. 4 - Prob. 4.101PCh. 4 - 4-102 Aspartame, an artificial sweetener used as a...Ch. 4 - 4-103 Caffeine, a central nervous system...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Please correct answer and don't used hand raitingarrow_forwardneed help please and thanks dont understand a-b Learning Goal: As discussed during the lecture, the enzyme HIV-1 reverse transcriptae (HIV-RT) plays a significant role for the HIV virus and is an important drug target. Assume a concentration [E] of 2.00 µM (i.e. 2.00 x 10-6 mol/l) for HIV-RT. Two potential drug molecules, D1 and D2, were identified, which form stable complexes with the HIV-RT. The dissociation constant of the complex ED1 formed by HIV-RT and the drug D1 is 1.00 nM (i.e. 1.00 x 10-9). The dissociation constant of the complex ED2 formed by HIV-RT and the drug D2 is 100 nM (i.e. 1.00 x 10-7). Part A - Difference in binding free eenergies Compute the difference in binding free energy (at a physiological temperature T=310 K) for the complexes. Provide the difference as a positive numerical expression with three significant figures in kJ/mol. The margin of error is 2%. Part B - Compare difference in free energy to the thermal energy Divide the…arrow_forwardPlease correct answer and don't used hand raitingarrow_forward
- Please correct answer and don't used hand raitingarrow_forwardCan you tell me if my answers are correctarrow_forwardBunsenite (NiO) crystallizes like common salt (NaCl), with a lattice parameter a = 4.177 Å. A sample of this mineral that has Schottky defects that are not supposed to decrease the volume of the material has a density of 6.67 g/cm3. What percentage of NiO molecules is missing? (Data: atomic weight of Ni: 58.7; atomic weight of O: 16).arrow_forward
- A sample of aluminum (face-centered cubic - FCC) has a density of 2.695 mg/m3 and a lattice parameter of 4.04958 Å. Calculate the fraction of vacancies in the structure. (Atomic weight of aluminum: 26.981).arrow_forwardPlease correct answer and don't used hand raitingarrow_forwardPlease correct answer and don't used hand raitingarrow_forward
- Please correct answer and don't used hand raitingarrow_forwardWhich of the following species is a valid resonance structure of A? Use curved arrows to show how A is converted to any valid resonance structure. When a compound is not a valid resonance structurc of A, explain why not. Provide steps and tips on what to look for to understand how to solve and apply to other problems.arrow_forwardN IZ Check the box under each structure in the table that is an enantiomer of the molecule shown below. If none of them are, check the none of the above box under the table. Molecule 1 Molecule 2 HN Molecule 3 Х HN www. Molecule 4 Molecule 5 Molecule 6 none of the above NH NH Garrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Stoichiometry - Chemistry for Massive Creatures: Crash Course Chemistry #6; Author: Crash Course;https://www.youtube.com/watch?v=UL1jmJaUkaQ;License: Standard YouTube License, CC-BY
Bonding (Ionic, Covalent & Metallic) - GCSE Chemistry; Author: Science Shorts;https://www.youtube.com/watch?v=p9MA6Od-zBA;License: Standard YouTube License, CC-BY
General Chemistry 1A. Lecture 12. Two Theories of Bonding.; Author: UCI Open;https://www.youtube.com/watch?v=dLTlL9Z1bh0;License: CC-BY