
Bundle: Introduction to General, Organic and Biochemistry, 11th + OWLv2, 4 terms (24 months) Printed Access Card
11th Edition
ISBN: 9781305705159
Author: Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 4, Problem 4.64P
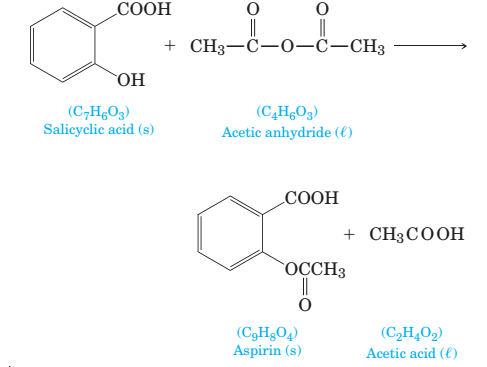
4-64 Aspirin is made by the reaction of salicylic acid with acetic anhydride. How many grams of aspirin are produced if 85.0 g of salicylic acid is treated with excess acetic anhydride?
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Solve the spectro
Don't used hand raiting and don't used Ai solution
Don't used hand raiting and don't used Ai solution
Chapter 4 Solutions
Bundle: Introduction to General, Organic and Biochemistry, 11th + OWLv2, 4 terms (24 months) Printed Access Card
Ch. 4.2 - Problem 4-1 Following is an unbalanced equation...Ch. 4.2 - Problem 4-2 Balance this equation:Ch. 4.2 - Prob. 4.3PCh. 4.3 - Problem 4-4 When a solution of copper(II)...Ch. 4.4 - Problem 4-5 In each equation, identify the...Ch. 4.5 - Problem 4-6 What is (a) the molecular weight of...Ch. 4.6 - Prob. 4.7PCh. 4.6 - Problem 4-8 We wish to weigh 2.84 mol of sodium...Ch. 4.6 - Problem 4-9 How many moles of C atoms, H atoms,...Ch. 4.6 - Problem 4-10 How many moles of copper(I) ions,...
Ch. 4.6 - Prob. 4.11PCh. 4.7 - Prob. 4.12PCh. 4.7 - Prob. 4.13PCh. 4.7 - Problem 4-14 Ethanol is produced industrially by...Ch. 4.7 - Prob. 4.15PCh. 4.7 - Prob. 4.16PCh. 4 - 4-17 Balance each equation.Ch. 4 - 4-18 Balance each equation.Ch. 4 - Prob. 4.19PCh. 4 - 4-20 Calcium oxide is prepared by heating...Ch. 4 - 4-21 The brilliant white light in some firework...Ch. 4 - Prob. 4.22PCh. 4 - 4-23 When solid carbon burns in a limited supply...Ch. 4 - Prob. 4.24PCh. 4 - 4-25 In the chemical test for arsenic, the gas...Ch. 4 - Prob. 4.26PCh. 4 - Prob. 4.27PCh. 4 - 4-28 Answer true or false. (a) A net ionic...Ch. 4 - 4-29 Balance these net ionic equations. (a)...Ch. 4 - 4-30 In the equation (a) Identify the spectator...Ch. 4 - 4-31 Predict whether a precipitate will form when...Ch. 4 - 4-32 When a solution of ammonium chloride is added...Ch. 4 - 4-33 When a solution of hydrochloric acid, HCl, is...Ch. 4 - Prob. 4.34PCh. 4 - Prob. 4.35PCh. 4 - 4-36 Using the solubility generalizations given in...Ch. 4 - 4-37 Answer true or false. (a) When a substance is...Ch. 4 - Prob. 4.38PCh. 4 - Prob. 4.39PCh. 4 - Prob. 4.40PCh. 4 - Prob. 4.41PCh. 4 - 4-42 Calculate the formula weight of: (a) KCl (b)...Ch. 4 - 4-43 Calculate the molecular weight of: (a)...Ch. 4 - 4-44 Answer true or false. (a) The mole is a...Ch. 4 - 4-45 Calculate the number of moles in: (a) 32 g of...Ch. 4 - 4-46 Calculate the number of grams in: (a) 1.77...Ch. 4 - 4-47 Calculate the number of moles of: (a) O atoms...Ch. 4 - 4-48 Calculate the number of moles of: (a) S2-...Ch. 4 - 4-49 Calculate the number of: (a) nitrogen atoms...Ch. 4 - 4-50 How many molecules are in each of the...Ch. 4 - 4-51 What is the mass in grams of each number of...Ch. 4 - 4-52 The molecular weight of hemoglobin is about...Ch. 4 - 4-53 A typical deposit of cholesterol, C27H46O, in...Ch. 4 - 4-54 Answer true or false. (a) Stoichiometry is...Ch. 4 - 4-55 For the reaction: (a) How many moles of N2...Ch. 4 - 4-56 Magnesium reacts with sulfuric acid according...Ch. 4 - 4-57 Chloroform, CHCl3, is prepared industrially...Ch. 4 - 4-58 At one time, acetaldehyde was prepared...Ch. 4 - 4-59 Chlorine dioxide, ClO2, is used for bleaching...Ch. 4 - 4-60 Ethanol, C2H6O, is added to gasoline to...Ch. 4 - 4-61 In photosynthesis, green plants convert CO2...Ch. 4 - 4-62 Iron ore is converted to iron by heating it...Ch. 4 - Prob. 4.63PCh. 4 - 4-64 Aspirin is made by the reaction of salicylic...Ch. 4 - 4-65 Suppose the preparation of aspirin from...Ch. 4 - 4-66 Benzene reacts with bromine to produce...Ch. 4 - 4-67 Ethyl chloride is prepared by the reaction of...Ch. 4 - 4-68 Diethyl ether is made from ethanol according...Ch. 4 - Prob. 4.69PCh. 4 - Prob. 4.70PCh. 4 - 4-71 Which of these reactions are exothermic, and...Ch. 4 - Prob. 4.72PCh. 4 - Prob. 4.73PCh. 4 - Prob. 4.74PCh. 4 - Prob. 4.75PCh. 4 - Prob. 4.76PCh. 4 - 4-77 To convert 1 mol of iron(III) oxide to its...Ch. 4 - 4-78 (Chemical Connections 4A) How does fluoride...Ch. 4 - Prob. 4.79PCh. 4 - Prob. 4.80PCh. 4 - 4-81 (Chemical Connections 4C) Balance the lithium...Ch. 4 - 4-82 When gaseous dinitrogen pentoxide, N2O5, is...Ch. 4 - Prob. 4.83PCh. 4 - Prob. 4.84PCh. 4 - Prob. 4.85PCh. 4 - 4-86 When an aqueous solution of Na3PO4 is added...Ch. 4 - Prob. 4.87PCh. 4 - 4-88 Chlorophyll, the compound responsible for the...Ch. 4 - 4-89 If 7.0 kg of is added to 11.0 kg of to form...Ch. 4 - 4-90 Lead(lI) nitrate and aluminum chloride react...Ch. 4 - 4-91 Assume that the average red blood cell has a...Ch. 4 - 4-92 Reaction of pentane, C5H12, with oxygen, O2,...Ch. 4 - 4-93 Ammonia is prepared industrially by the...Ch. 4 - 4-94 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) is...Ch. 4 - Prob. 4.95PCh. 4 - Prob. 4.96PCh. 4 - Prob. 4.97PCh. 4 - Prob. 4.98PCh. 4 - Prob. 4.99PCh. 4 - Prob. 4.100PCh. 4 - Prob. 4.101PCh. 4 - 4-102 Aspartame, an artificial sweetener used as a...Ch. 4 - 4-103 Caffeine, a central nervous system...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 2. 200 LOD For an unknown compound with a molecular ion of 101 m/z: a. Use the molecular ion to propose at least two molecular formulas. (show your work) b. What is the DU for each of your possible formulas? (show your work) C. Solve the structure and assign each of the following spectra. 8 6 4 2 (ppm) 150 100 50 ō (ppm) 4000 3000 2000 1500 1000 500 HAVENUMBERI-11arrow_forwardComplete the spectroscopy with structurearrow_forwardComplete the spectroscopy with structurearrow_forward
- Given the following concentrations for a system, calculate the value for the reaction quotient: Cl2(g)+ CS2(g) ⇌ CCl4(g)+ S2Cl2(g) Cl2 = 31.1 atm CS2 = 91.2 atm CCl4 = 2.12 atm S2Cl2 = 10.4 atmarrow_forwardMatch each chemical or item with the proper disposal or cleanup mwthod, Not all disposal and cleanup methods will be labeled. Metal sheets C, calcium, choroide solutions part A, damp metal pieces Part B, volumetric flask part A. a.Return to correct lables”drying out breaker. Place used items in the drawer.: Rinse with deionized water, dry as best you can, return to instructor. Return used material to the instructor.: Pour down the sink with planty of running water.: f.Pour into aqueous waste container. g.Places used items in garbage.arrow_forwardWrite the equilibrium constant expression for the following reaction: HNO2(aq) + H2O(l) ⇌ H3O+(aq) + NO2-(aq)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning
Bonding (Ionic, Covalent & Metallic) - GCSE Chemistry; Author: Science Shorts;https://www.youtube.com/watch?v=p9MA6Od-zBA;License: Standard YouTube License, CC-BY
Stoichiometry - Chemistry for Massive Creatures: Crash Course Chemistry #6; Author: Crash Course;https://www.youtube.com/watch?v=UL1jmJaUkaQ;License: Standard YouTube License, CC-BY