
Practice Problem CONCEPTUALIZE
CONCEPTUALIZE
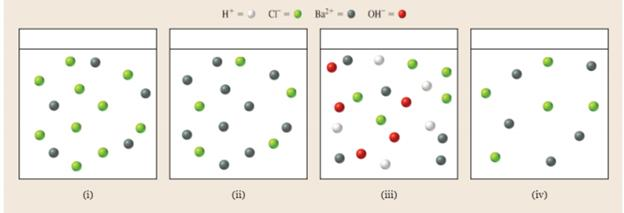
Which diagram best represents the ions in solution at the equivalence point in the titration of
Want to see the full answer?
Check out a sample textbook solution
Chapter 4 Solutions
CHEMISTRY >CUSTOM<
Additional Science Textbook Solutions
Physics for Scientists and Engineers: A Strategic Approach, Vol. 1 (Chs 1-21) (4th Edition)
HUMAN ANATOMY
Physics of Everyday Phenomena
Fundamentals Of Thermodynamics
Essentials of Human Anatomy & Physiology (12th Edition)
- QUESTION 19 What is the pH of a buffer made by combining 45.0 mL of 0.150 M nitrous acid and 20.0 mL of 0.175 M sodium nitrite? The Ka of nitrous acid, HNO2, is 4.5x10-4. [A"] pH = pK +log [HA] O 3.06 3.28 O 3.42 O 3.64arrow_forwardWhich of the following aqueous solutions are good buffer systems? (Select all that apply.) □ 0.12 M hydrocyanic acid + 0.10 M sodium cyanide □ 0.22 M hydroiodic acid + 0.19 M potassium iodide O 0.25 M ammonia + 0.35 M barium hydroxide □ 0.15 M sodium hydroxide + 0.26 M sodium bromide ☐ 0.37 M barium perchlorate + 0.20 M potassium perchloratearrow_forwardWhich of the following aqueous solutions are good buffer systems? (Select all that apply.) 0.18 M hydrocyanic acid + 0.17 M sodium cyanide 0.27 M nitric acid + 0.21 M potassium nitrate 0.28 M ammonia + 0.39 M sodium hydroxide 0.17 M barium hydroxide + 0.20 M barium chloride 0.38 M calcium iodide + 0.26 M barium iodidearrow_forward
- Which of the following aqueous solutions are buffer solutions? (Select all that apply.) 0.27 M HBr + 0.22 M KBr 0.13 M HF + 0.19 M NaF 0.38 M HClO + 0.27 M NaClO 0.33 M NH4Br + 0.39 M NH3 0.19 M Br(OH)2 + 0.22 M BaBr2arrow_forwardWhich of the following aqueous solutions are good buffer systems? (Select all that apply.) 0.30 M hydroiodic acid + 0.17 M sodium iodide O 0.30 M ammonia + 0.39 M ammonium bromide 0.36 M barium bromide + 0.22 M potassium bromide O 0.14 M hypochlorous acid + 0.20 M perchloric acid O 0.18 M potassium hydroxide + 0.23 M potassium chloridearrow_forwardWhich of the following aqueous solutions are good buffer systems? (Select all that apply.) 0.16 M hydrocyanic acid + 0.15 M potassium cyanide 0.22 M nitric acid + 0.18 M sodium nitrate 0.29 M ammonia + 0.33 M sodium hydroxide 0.13 M calcium hydroxide + 0.29 M calcium bromide 0.39 M sodium nitrate + 0.24 M calcium nitratearrow_forward
- Which of the following aqueous solutions are good buffer systems? (Select all that apply.) □ 0.20 M hypochlorous acid + 0.12 M potassium hypochlorite O 0.26 M ammonium bromide + 0.38 M ammonia □ 0.24 M hydroiodic acid + 0.16 M potassium iodide O 0.33 M hydrofluoric acid + 0.22 M potassium fluoride 0.14 M potassium acetate + 0.28 M acetic acidarrow_forwardWhich of the following aqueous solutions are good buffer systems? (Select all that apply.) 0.22 M hydrocyanic acid + 0.10 M sodium cyanide 0.27 M perchloric acid + 0.20 M sodium perchlorate 0.28 M ammonium nitrate + 0.38 M ammonia 0.15 M barium hydroxide + 0.29 M barium bromide 0.34 M hypochlorous acid + 0.20 M potassium hypochloritearrow_forwardWhich of the following aqueous solutions are good buffer systems? (Select all that apply.) 0.14 M hydrofluoric acid + 0.12 M sodium fluoride 0.27 M nitric acid + 0.18 M potassium nitrate 0.28 M ammonium nitrate + 0.30 M ammonia 0.12 M sodium hydroxide + 0.28 M sodium bromide 0.31 M hydrocyanic acid + 0.25 M sodium cyanide Submit Answer Retry Entire Group 7 more group attempts remainingarrow_forward
- Which of the following aqueous solutions are good buffer systems? (Select all that apply.) 0.19 M acetic acid + 0.19 M sodium acetate 0.22 M perchloric acid + 0.23 M sodium perchlorate 0.30 M ammonia + 0.36 M calcium hydroxide 0.17 M sodium hydroxide + 0.29 M sodium chloride 0.38 M sodium perchlorate + 0.26 M potassium perchloratearrow_forwardWhich of the following aqueous solutions are good buffer systems? (Select all that apply.) 0.17 M hydrocyanic acid + 0.17 M potassium cyanide O 0.30 M perchloric acid + 0.20 M potassium perchlorate 0.26 M ammonium nitrate + 0.30 M ammonia 0.19 M sodium hydroxide + 0.20 M sodium chloride 0.36 M hypochlorous acid + 0.26 M potassium hypochloritearrow_forwardWhich of the following aqueous solutions are good buffer systems? (Select all that apply.) 0.24 M hydroiodic acid + 0.19 M sodium iodide 0.35 M ammonium bromide + 0.32 M ammonia 0.35 M acetic acid + 0.29 M sodium acetate 0.10 M hypochlorous acid + 0.11 M sodium hypochlorite 0.18 M sodium cyanide + 0.28 M hydrocyanic acid Submit Answer Retry Entire Group 7 more group attempts remainingarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





