
(a)
Interpretation:
Chair conformations of given compound should be drawn and most stable conformer is needed to be predicted between them and stable conformation at equilibrium also needed to be find.
Concept introduction:
Chair conformer: chair conformer is a stable conformer for cyclohexane compound. In this chair conformer two positions are important for substitutions one is equatorial and other one axial position. Axial positions are parallel to the axis of ring while equatorial positions are perpendicular to the axis of the ring.
Example:
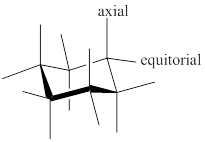
After ring flip of the conformer, axial position becomes equatorial and equatorial position becomes axial.
Stereochemistry conversions in chair conformation are shown below.
| Substituent position | cis | trans |
| 1,2 position | (a,e) or (e,a) | (a,a) or (e,e) |
| 1,3 position | (a,a) or (e,e) | (a,e) or (e,a) |
| 1,4 position | (a,e) or (e,a) | (a,a) or (e,e) |
(b)
Interpretation:
Chair conformations of given compound should be drawn and most stable conformer is needed to be predicted between them and stable conformation at equilibrium also needed to be find.
Concept introduction:
Chair conformer: chair conformer is a stable conformer for cyclohexane compound. In this chair conformer two positions are important for substitutions one is equatorial and other one axial position. Axial positions are parallel to the axis of ring while equatorial positions are perpendicular to the axis of the ring.
Example:

After ring flip of the conformer, axial position becomes equatorial and equatorial position becomes axial.
Stereochemistry conversions in chair conformation are shown below.
| Substituent position | cis | trans |
| 1,2 position | (a,e) or (e,a) | (a,a) or (e,e) |
| 1,3 position | (a,a) or (e,e) | (a,e) or (e,a) |
| 1,4 position | (a,e) or (e,a) | (a,a) or (e,e) |
(c)
Interpretation:
Chair conformations of given compound should be drawn and most stable conformer is needed to be predicted between them and stable conformation at equilibrium also needed to be find.
Concept introduction:
Chair conformer: chair conformer is a stable conformer for cyclohexane compound. In this chair conformer two positions are important for substitutions one is equatorial and other one axial position. Axial positions are parallel to the axis of ring while equatorial positions are perpendicular to the axis of the ring.
Example:

After ring flip of the conformer, axial position becomes equatorial and equatorial position becomes axial.
Stereochemistry conversions in chair conformation are shown below.
| Substituent position | cis | trans |
| 1,2 position | (a,e) or (e,a) | (a,a) or (e,e) |
| 1,3 position | (a,a) or (e,e) | (a,e) or (e,a) |
| 1,4 position | (a,e) or (e,a) | (a,a) or (e,e) |
Trending nowThis is a popular solution!

Chapter 4 Solutions
EBK ORGANIC CHEMISTRY-STUD.SOLNS.MAN+SG
- How many chiral centers are there in the following molecule? HO 0 1 ○ 2 ♡ 4 'N'arrow_forwardThe following chemical structure represents a molecule of what molecular formula?arrow_forwardWhich region(s) of the following phospholipid is/are hydrophobic? RO I hydro-water phobic-dislikes = Hydrophobic dislikes water ○ I only Il only I and III only II and IV only O II, III, and IV only III || IVarrow_forward
- Given the following data, determine the order of the reaction with respect to H2. H2(g) + 21Cl(g) → I2(g) + 2HCl(g) Experiment [H2] (torr) [ICI] (torr) Rate (M/s) 1 250 325 0.266 2 250 81 0.0665 3 50 325 0.266arrow_forwardWhich one of the following molecules is chiral? H- NH₂ H3C དང་།་ OH H HO H₂N HO- -H CHO -OH H HO- OH H- -H CH₂OH OHarrow_forwardThe structure of an unsaturated phospholipid is shown below. Which region of the molecule is most hydrophilic ? H₂N-CH₂ H₂C IV CH3 CH3 hydro-water philic-likes = Hydrophilic likes water ○ IV All regions are equally hydrophilic. IIIarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





