
(a)
Interpretation:
Chair conformations of given compound should be drawn and most stable conformer is needed to be predicted between them.
Concept introduction:
Chair conformer: chair conformer is a stable conformer for cyclohexane compound. In this chair conformer two positions are important for substitutions one is equatorial and other one axial position. Axial positions are parallel to the axis of ring while equatorial positions are perpendicular to the axis of the ring.
Example:
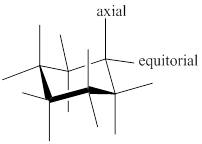
After ring flip of the conformer, axial position becomes equatorial and equatorial position becomes axial.
Stereochemistry conversions in chair conformation are shown below.
| Substituent position | cis | trans |
| 1,2 position | (a,e) or (e,a) | (a,a) or (e,e) |
| 1,3 position | (a,a) or (e,e) | (a,e) or (e,a) |
| 1,4 position | (a,e) or (e,a) | (a,a) or (e,e) |
(b)
Interpretation:
Chair conformations of given compound should be drawn and most stable conformer is needed to be predicted between them.
Concept introduction:
Chair conformer: chair conformer is a stable conformer for cyclohexane compound. In this chair conformer two positions are important for substitutions one is equatorial and other one axial position. Axial positions are parallel to the axis of ring while equatorial positions are perpendicular to the axis of the ring.
Example:

After ring flip of the conformer, axial position becomes equatorial and equatorial position becomes axial.
Stereochemistry conversions in chair conformation are shown below.
| Substituent position | cis | trans |
| 1,2 position | (a,e) or (e,a) | (a,a) or (e,e) |
| 1,3 position | (a,a) or (e,e) | (a,e) or (e,a) |
| 1,4 position | (a,e) or (e,a) | (a,a) or (e,e) |
(c)
Interpretation:
Chair conformations of given compound should be drawn and most stable conformer is needed to be predicted between them.
Concept introduction:
Chair conformer: chair conformer is a stable conformer for cyclohexane compound. In this chair conformer two positions are important for substitutions one is equatorial and other one axial position. Axial positions are parallel to the axis of ring while equatorial positions are perpendicular to the axis of the ring.
Example:

After ring flip of the conformer, axial position becomes equatorial and equatorial position becomes axial.
Stereochemistry conversions in chair conformation are shown below.
| Substituent position | cis | trans |
| 1,2 position | (a,e) or (e,a) | (a,a) or (e,e) |
| 1,3 position | (a,a) or (e,e) | (a,e) or (e,a) |
| 1,4 position | (a,e) or (e,a) | (a,a) or (e,e) |
(d)
Interpretation:
Chair conformations of given compound should be drawn and most stable conformer is needed to be predicted between them.
Concept introduction:
Chair conformer: chair conformer is a stable conformer for cyclohexane compound. In this chair conformer two positions are important for substitutions one is equatorial and other one axial position. Axial positions are parallel to the axis of ring while equatorial positions are perpendicular to the axis of the ring.
Example:

After ring flip of the conformer, axial position becomes equatorial and equatorial position becomes axial.
Stereochemistry conversions in chair conformation are shown below.
| Substituent position | cis | trans |
| 1,2 position | (a,e) or (e,a) | (a,a) or (e,e) |
| 1,3 position | (a,a) or (e,e) | (a,e) or (e,a) |
| 1,4 position | (a,e) or (e,a) | (a,a) or (e,e) |
Want to see the full answer?
Check out a sample textbook solution
Chapter 4 Solutions
EBK ORGANIC CHEMISTRY-STUD.SOLNS.MAN+SG
- For a particular hypothetical reaction, A+B →2C, the value of AG° is -125 kJ/mol. What is the value of AG for this reaction at 35°C when [A] = 0.10 M, [B] = 0.05 M, and [C] = 2.0 × 10¹ M?arrow_forwardIn an experiment, 74.3 g of metallic copper was heated to 100.0°C and then quickly dropped into 200.0 mL of water in a calorimeter. The heat capacity of the calorimeter with the water was 875 J/°C. The initial temperature of the calorimeter was 27.5°C, and the final temperature after addition of the metal was 29.8°C. What is the value of the molar heat capacity of copper?arrow_forwardThe Haber-Bosch process permits the direct conversion of molecular nitrogen to ammonia, which can be used in large-scale fertilizer production. Given the balanced Haber-Bosch reaction and using the bond energies in the table below, estimate the enthalpy change associated with the reaction. N2(g) + 3H2(g) → 2NH3(g) Bond N=N N = N Energy (kJ/mol) 941 418 N-N H-H N-H 163 435 388arrow_forward
- Benzoic acid is used to determine the heat capacity of bomb calorimeters because it can be obtained in pure form and its energy of combustion is known very accurately (−26.43 kJ/g). Determine the heat capacity of a calorimeter that had a temperature increase of 9.199°C when 3.500 g of benzoic acid was used.arrow_forwardGiven the standard enthalpies of formation for the following substances, determine the reaction enthalpy for the following reaction. 2N2H4(g) + 2NO2(g) → 3N2(g) + 4H2O(g) AHrxn ? kJ Substance AH in kJ/mol N2H4(g) +95.4 NO2(g) +33.1 H2O(g) -241.8arrow_forwardIf 7.3 kJ of energy are required to change the temperature of water from 5.0 to 70.0, what was the volume of water? (cs = 4.184 J/(g ⋅ ), d = 1.00 g/mL)arrow_forward
- BALANCE CHEMICAL REACTIONarrow_forwardPredict the product(s) of the following reactions. If no reaction, write "NR". a) Cl₂ FeCl3 e) HNO3 H2SO4 b) NO2 CI. HNO3 f) Br Br2 OH H2SO4 HO3S. FeBr3 c) Cl2 g) FeCl3 F d) O₂N Br2 FeBr3 O₂N OH HNO3 CH3 H2SO4arrow_forwardulating the pH salt solution Calculate the pH at 25 °C of a 0.75M solution of anilinium chloride (C6H5NH3C1). Note that aniline (C6H5NH2) is a weak base with a pK of 4.87. Round your answer to 1 decimal place. pH = ☐ ☑ ⑤ ? olo 18 Ararrow_forward
- I apologize, but the app is not allowing me to post the other 4 pictures of the thermodynamics chart. But I believe the values are universal. Please help!arrow_forwardCalculating the pH of a salt solution Calculate the pH at 25 °C of a 0.29M solution of potassium butanoate (KC3H,CO2). Note that butanoic acid (HC3H,CO2) is a weak acid with a pKa of 4.82. Round your answer to 1 decimal place. pH = -0 Х olo 18 Ararrow_forward: At a certain temperature, the equilibrium constant K for the following reaction is 1.58 × 10-12 N2(g) + O2(g) = 2 NO(g) Use this information to complete the following table. Suppose a 38. L reaction vessel is filled with 0.93 mol of N2 and 0.93 mol of O2. What can you say about the composition of the mixture in the vessel at equilibrium? There will be very little N2 and O2. There will be very little NO. What is the equilibrium constant for the following reaction? Be sure your answer has the correct number of significant digits. 2 NO(g) N2(9)+02(9) What is the equilibrium constant for the following reaction? Be sure your answer has the correct number of significant digits. 3 N2(9)+302(g) 6 NO(g) Neither of the above is true. K = ☐ K = ☐ ☐ X10 Х D ? 000 18 Ar Barrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





