
Pearson eText for Essential Organic Chemistry -- Instant Access (Pearson+)
3rd Edition
ISBN: 9780137533268
Author: Paula Bruice
Publisher: PEARSON+
expand_more
expand_more
format_list_bulleted
Question
Chapter 4, Problem 64P
Interpretation Introduction
Interpretation:
The reason should be explained for the given compound has four stereoisomers when it has only one asymmetric center.
Concept introduction:
The total number of stereoisomers can be calculated by using following formula,
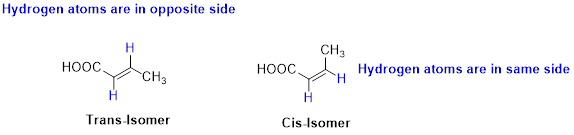
Cis–trans isomerism (or) geometric isomerism or configurational isomerism:
The two similar groups (or higher priority groups) are in same side in double bond of
Example:
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
None
Please correct answer and don't use hand rating
Describe the structural differences between iso- and heteropolyacids.
Chapter 4 Solutions
Pearson eText for Essential Organic Chemistry -- Instant Access (Pearson+)
Ch. 4.1 - Draw the cis and trans isomers for the following:...Ch. 4.1 - Prob. 4PCh. 4.1 - Prob. 5PCh. 4.1 - Prob. 6PCh. 4.2 - Prob. 7PCh. 4.2 - Tamoxifen slows the growth of some breast tumors...Ch. 4.2 - Draw and label the E and Z isomers for each of the...Ch. 4.2 - Prob. 10PCh. 4.2 - Name each of the following:Ch. 4.2 - Draw the structure of (Z)-2,3-dimethyl-3-heptene.
Ch. 4.3 - Prob. 13PCh. 4.4 - Prob. 14PCh. 4.5 - Prob. 16PCh. 4.6 - Prob. 17PCh. 4.7 - Assign relative priorities to the groups or atoms...Ch. 4.7 - Name the following:Ch. 4.7 - Prob. 22PCh. 4.7 - Draw a perspective formula for each of the...Ch. 4.8 - Prob. 24PCh. 4.8 - Prob. 27PCh. 4.9 - Prob. 28PCh. 4.9 - Prob. 29PCh. 4.9 - Prob. 30PCh. 4.10 - Prob. 31PCh. 4.10 - Prob. 32PCh. 4.10 - Prob. 33PCh. 4.11 - Prob. 34PCh. 4.11 - a. Draw the stereoisomers of...Ch. 4.11 - Prob. 37PCh. 4.11 - Prob. 38PCh. 4.12 - Which of the following compounds has a...Ch. 4.12 - Draw all the stereoisomers for each of the...Ch. 4.12 - Limonene exists as two different stereoisomers....Ch. 4 - a. Draw three constitutional isomers with...Ch. 4 - Which of the following have an asymmetric center?...Ch. 4 - Prob. 45PCh. 4 - Prob. 46PCh. 4 - Of all the possible cyclooctanes that have one...Ch. 4 - Prob. 48PCh. 4 - Prob. 49PCh. 4 - Prob. 50PCh. 4 - Prob. 51PCh. 4 - Prob. 52PCh. 4 - Draw the stereoisomers of 2,4-dichlorohexane....Ch. 4 - Prob. 54PCh. 4 - Prob. 55PCh. 4 - Prob. 56PCh. 4 - Prob. 57PCh. 4 - Prob. 58PCh. 4 - Prob. 59PCh. 4 - Prob. 60PCh. 4 - Prob. 61PCh. 4 - Prob. 62PCh. 4 - Draw structures for each of the following: a....Ch. 4 - Prob. 64PCh. 4 - a. Draw all the isomers with molecular formula...Ch. 4 - Prob. 66PCh. 4 - Prob. 67PCh. 4 - Prob. 68PCh. 4 - Chloramphenicol is a broad-spectrum antibiotic...
Knowledge Booster
Similar questions
- What is the pH of the Tris buffer after the addition of 10 mL of 0.01M NaOH? How would I calculate this?arrow_forwardWhy do isopolianions form polymeric species with a defined molecular weight? What does it depend on?arrow_forwardWhat are isopolianions? Describe the structural unit of isopolianions.arrow_forward
- Justify the polymerization of vanadates VO43-, as a function of concentration and pH.arrow_forwardWhat is the preparation of 500 mL of 100mM MOPS buffer (pH=7.5) starting with 1 M MOPS and 1 M NaOH? How would I calculate the math?arrow_forwardIndicate the correct option.a) Isopolianions are formed around metallic atoms in a low oxidation state.b) Non-metals such as N, S, C, Cl, ... give rise to polyacids (oxygenated).c) Both are incorrect.arrow_forward
- 14. Which one of the compounds below is the major organic product obtained from the following series of reactions? Br OH OH CH3O™ Na+ H*, H₂O SN2 HO OH A B C D 0 Earrow_forwardWavelength (nm) I'm not sure what equation I can come up with other than the one generated with my graph. Can you please show me the calculations that were used to find this equation? Give an equation that relates energy to wavelength. Explain how you arrived at your equation. Wavelength Energy (kJ/mol) (nm) 350 341.8 420 284.8 470 254.5 530 225.7 580 206.3 620 192.9 700 170.9 750 159.5 Energy vs. Wavelength (Graph 1) 400 350 y=-0.4367x+470.82 300 250 200 150 100 50 O 0 100 200 300 400 500 600 700 800 Energy (kJ/mol)arrow_forward5. Draw molecular orbital diagrams for superoxide (O2¯), and peroxide (O2²-). A good starting point would be MO diagram for O2 given in your textbook. Then: a) calculate bond orders in superoxide and in peroxide; indicate which species would have a stronger oxygen-oxygen bond; b) indicate which species would be a radical. (4 points)arrow_forward
- 16. Which one of the compunds below is the final product of the reaction sequence shown here? عملاء .OH Br. (CH3)2CH-C=C H+,H,O 2 mol H2, Pt A OH B OH D OH E OH C OHarrow_forwardIndicate whether any of the two options is correct.a) The most common coordination structure for isopolianions is the prismb) Heteropolianions incorporate alkaline cations into their structuresarrow_forwardPlease correct answer and don't use hand ratingarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:9781305577190
Author:Kenneth L. Williamson, Katherine M. Masters
Publisher:Brooks Cole
