
Bundle: Chemistry: An Atoms First Approach, Loose-leaf Version, 2nd + OWLv2 with Student Solutions Manual, 4 terms (24 months) Printed Access Card
2nd Edition
ISBN: 9781337086431
Author: Steven S. Zumdahl, Susan A. Zumdahl
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 4, Problem 60E
The antibiotic thiarubin-A was discovered by studying the feeding habits of wild chimpanzees in Tanzania. The structure for thiarubin-A is
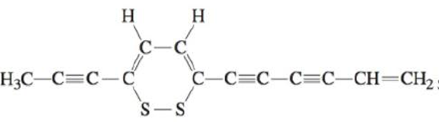
a. Complete the Lewis structure, showing all lone pairs of electrons.
b. Indicate the hybrid orbitals used by the carbon and sulfur atoms in thiarubin-A.
c. How many σ and π bonds are present in this molecule?
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Please help me solve this homework problem
Please help me answer this homework question
Calculating standard reaction free energy from standard reduction...
Using standard reduction potentials from the ALEKS Data tab, calculate the standard reaction free energy AG° for the following redox reaction.
Be sure your answer has the correct number of significant digits.
3+
H2(g)+2OH¯ (aq) + 2Fe³+ (aq) → 2H₂O (1)+2Fe²+ (aq)
0
kJ
x10
Х
?
olo
18
Ar
Chapter 4 Solutions
Bundle: Chemistry: An Atoms First Approach, Loose-leaf Version, 2nd + OWLv2 with Student Solutions Manual, 4 terms (24 months) Printed Access Card
Ch. 4 - Explain the main postulate of the VSEPR model....Ch. 4 - Explain why CF4 and Xef4 are nonpolar compounds...Ch. 4 - Consider the following compounds: CO2, SO2, KrF2,...Ch. 4 - Prob. 4RQCh. 4 - What hybridization is required for central atoms...Ch. 4 - Prob. 6RQCh. 4 - Prob. 7RQCh. 4 - What are molecular orbitals? How do they compare...Ch. 4 - Explain the difference between the and MOs for...Ch. 4 - Prob. 3ALQ
Ch. 4 - Which of the following would you expect to be more...Ch. 4 - Arrange the following molecules from most to least...Ch. 4 - Which is the more correct statement: The methane...Ch. 4 - Prob. 7ALQCh. 4 - Prob. 8ALQCh. 4 - Which of the following statements is/are true?...Ch. 4 - Give one example of a compound having a linear...Ch. 4 - In the hybrid orbital model, compare and contrast ...Ch. 4 - Prob. 13QCh. 4 - Prob. 14QCh. 4 - Prob. 15QCh. 4 - Prob. 16QCh. 4 - Compare and contrast bonding molecular orbitals...Ch. 4 - Prob. 18QCh. 4 - Why does the molecular orbital model do a better...Ch. 4 - The three NO bonds in NO3 are all equivalent in...Ch. 4 - Predict the molecular structure (including bond...Ch. 4 - Predict the molecular structure (including bond...Ch. 4 - Predict the molecular structure and bond angles...Ch. 4 - Prob. 24ECh. 4 - Prob. 25ECh. 4 - Two variations of the octahedral geometry (see...Ch. 4 - Predict the molecular structure (including bond...Ch. 4 - Predict the molecular structure (including bond...Ch. 4 - State whether or not each of the following has a...Ch. 4 - The following electrostatic potential diagrams...Ch. 4 - Which of the molecules in Exercises 21 and 22 have...Ch. 4 - Which of the molecules in Exercises 27 and 28 have...Ch. 4 - Write Lewis structures and predict the molecular...Ch. 4 - Write Lewis structures and predict whether each of...Ch. 4 - Consider the following Lewis structure where E is...Ch. 4 - Consider the following Lewis structure where E is...Ch. 4 - The molecules BF3, CF4, CO2, PF5, and SF6 are all...Ch. 4 - Two different compounds have the formula XeF2Cl2....Ch. 4 - Use the localized electron model to describe the...Ch. 4 - Use the localized electron model to describe the...Ch. 4 - Use the localized electron model to describe the...Ch. 4 - Use the localized electron model to describe the...Ch. 4 - The space-filling models of ethane and ethanol are...Ch. 4 - The space-filling models of hydrogen cyanide and...Ch. 4 - Prob. 45ECh. 4 - Prob. 46ECh. 4 - Prob. 47ECh. 4 - Give the expected hybridization of the central...Ch. 4 - For each of the following molecules, write the...Ch. 4 - For each of the following molecules or ions that...Ch. 4 - Prob. 51ECh. 4 - The allene molecule has the following Lewis...Ch. 4 - Indigo is the dye used in coloring blue jeans. The...Ch. 4 - Prob. 54ECh. 4 - Prob. 55ECh. 4 - Many important compounds in the chemical industry...Ch. 4 - Two molecules used in the polymer industry are...Ch. 4 - Hot and spicy foods contain molecules that...Ch. 4 - One of the first drugs to be approved for use in...Ch. 4 - The antibiotic thiarubin-A was discovered by...Ch. 4 - Prob. 61ECh. 4 - Sketch the molecular orbital and label its type (...Ch. 4 - Prob. 63ECh. 4 - Which of the following are predicted by the...Ch. 4 - Prob. 65ECh. 4 - Prob. 66ECh. 4 - Prob. 67ECh. 4 - Using the molecular orbital model to describe the...Ch. 4 - Prob. 69ECh. 4 - A Lewis structure obeying the octet rule can be...Ch. 4 - Using the molecular orbital model, write electron...Ch. 4 - Using the molecular orbital model, write electron...Ch. 4 - In which of the following diatomic molecules would...Ch. 4 - In terms of the molecular orbital model, which...Ch. 4 - Prob. 75ECh. 4 - Show how a hydrogen 1s atomic orbital and a...Ch. 4 - Use Figs. 4-54 and 4-55 to answer the following...Ch. 4 - The diatomic molecule OH exists in the gas phase....Ch. 4 - Prob. 79ECh. 4 - Describe the bonding in NO+, NO, and NO, using...Ch. 4 - Describe the bonding in the O3 molecule and the...Ch. 4 - Prob. 82ECh. 4 - Prob. 83AECh. 4 - Vitamin B6 is an organic compound whose deficiency...Ch. 4 - Two structures can be drawn for cyanuric acid: a....Ch. 4 - Prob. 86AECh. 4 - What do each of the following sets of...Ch. 4 - Aspartame is an artificial sweetener marketed...Ch. 4 - Prob. 89AECh. 4 - The three most stable oxides of carbon are carbon...Ch. 4 - Prob. 91AECh. 4 - Which of the following molecules have net dipole...Ch. 4 - The strucrure of TeF5 is Draw a complete Lewis...Ch. 4 - Complete the following resonance structures for...Ch. 4 - Prob. 95AECh. 4 - Describe the bonding in the first excited state of...Ch. 4 - Using an MO energy-level diagram, would you expect...Ch. 4 - Show how a dxz. atomic orbital and a pz, atomic...Ch. 4 - What type of molecular orbital would result from...Ch. 4 - Consider three molecules: A, B, and C. Molecule A...Ch. 4 - Prob. 101CWPCh. 4 - Predict the molecular structure, bond angles, and...Ch. 4 - Draw the Lewis structures for SO2, PCl3, NNO, COS,...Ch. 4 - Draw the Lewis structures for TeCl4, ICl5, PCl5,...Ch. 4 - A variety of chlorine oxide fluorides and related...Ch. 4 - Pelargondin is the molecule responsible for the...Ch. 4 - Complete a Lewis structure for the compound shown...Ch. 4 - Prob. 108CWPCh. 4 - Consider the molecular orbital electron...Ch. 4 - Place the species B2+ , B2, and B2 in order of...Ch. 4 - The compound NF3 is quite stable, but NCl3 is very...Ch. 4 - Predict the molecular structure for each of the...Ch. 4 - Prob. 113CPCh. 4 - Cholesterol (C27liu;O) has the following...Ch. 4 - Cyanamide (H2NCN), an important industrial...Ch. 4 - As compared with CO and O2, CS and S2 are very...Ch. 4 - Prob. 117CPCh. 4 - Use the MO model to explain the bonding in BeH2....Ch. 4 - Prob. 119CPCh. 4 - Arrange the following from lowest to highest...Ch. 4 - Prob. 121CPCh. 4 - Prob. 122CPCh. 4 - Carbon monoxide (CO) forms bonds to a variety of...Ch. 4 - The space-filling model for benzoic acid, a food...Ch. 4 - As the bead engineer of your starship in charge of...Ch. 4 - A flask containing gaseous N2 is irradiated with...Ch. 4 - Determine the molecular structure and...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Calculating the pH of a weak base titrated with a strong acid An analytical chemist is titrating 184.2 mL of a 0.7800M solution of dimethylamine ((CH3) NH with a 0.3000M solution of HClO4. The pK₁ of dimethylamine is 3.27. Calculate the pH of the base solution after the chemist has added 424.1 mL of the HClO solution to it. 2 4 Note for advanced students: you may assume the final volume equals the initial volume of the solution plus the volume of HClO 4 solution added. Round your answer to 2 decimal places. pH = ☐ ☑ ? 000 18 Ar 1 Barrow_forwardUsing the Nernst equation to calculate nonstandard cell voltage A galvanic cell at a temperature of 25.0 °C is powered by the following redox reaction: MnO2 (s)+4H* (aq)+2Cr²+ (aq) → Mn²+ (aq)+2H₂O (1)+2Cr³+ (aq) + 2+ 2+ 3+ Suppose the cell is prepared with 7.44 M H* and 0.485 M Cr²+ in one half-cell and 7.92 M Mn² and 3.73 M Cr³+ in the other. Calculate the cell voltage under these conditions. Round your answer to 3 significant digits. ☐ x10 μ Х 5 ? 000 日。arrow_forwardCalculating standard reaction free energy from standard reduction... Using standard reduction potentials from the ALEKS Data tab, calculate the standard reaction free energy AG° for the following redox reaction. Be sure your answer has the correct number of significant digits. NO (g) +H₂O (1) + Cu²+ (aq) → HNO₂ (aq) +H* (aq)+Cu* (aq) kJ - ☐ x10 x10 olo 18 Ararrow_forward
- Calculating the pH of a weak base titrated with a strong acid b An analytical chemist is titrating 116.9 mL of a 0.7700M solution of aniline (C6H5NH2) with a 0.5300M solution of HNO3. The pK of aniline is 9.37. Calculate the pH of the base solution after the chemist has added 184.2 mL of the HNO 3 solution to it. Note for advanced students: you may assume the final volume equals the initial volume of the solution plus the volume of HNO3 solution added. Round your answer to 2 decimal places. pH = ☐ ☑ 5arrow_forwardQUESTION: Find the standard deviation for the 4 different groups 5.298 3.977 223.4 148.7 5.38 4.24 353.7 278.2 5.033 4.044 334.6 268.7 4.706 3.621 305.6 234.4 4.816 3.728 340.0 262.7 4.828 4.496 304.3 283.2 4.993 3.865 244.7 143.6 STDEV = STDEV = STDEV = STDEV =arrow_forwardQUESTION: Fill in the answers in the empty green boxes regarding 'Question 5: Calculating standard error of regression' *The images of the data showing 'coefficients for the standard curve' have been providedarrow_forward
- Using the Nernst equation to calculate nonstandard cell voltage Try Again Your answer is wrong. In addition to checking your math, check that you used the right data and DID NOT round any intermediate calculations. A galvanic cell at a temperature of 25.0 °C is powered by the following redox reaction: 2+ 2+ Sn²+ Ba(s) (aq) + Ba (s) Sn (s) + Ba²+ (aq) →>> Suppose the cell is prepared with 6.10 M Sn 2+ 2+ in one half-cell and 6.62 M Ba in the other. Calculate the cell voltage under these conditions. Round your answer to 3 significant digits. 1.71 V ☐ x10 ☑ 5 0/5 ? 00. 18 Ararrow_forwardQuestion: Find both the b (gradient) and a (y-intercept) value from the list of data below: (x1 -x̄) 370.5 (y1 - ȳ) 5.240 (x2 - x̄) 142.5 (y2 - ȳ) 2.004 (x3 - x̄) 28.5 (y3 - ȳ) 0.390 (x4 - x̄) -85.5 (y4 - ȳ) -1.231 (x5 - x̄) -199.5 (y5 - ȳ) -2.829 (x6 - x̄) -256.5 (y6 - ȳ) -3.575arrow_forwardCalculating standard reaction free energy from standard reduction... Using standard reduction potentials from the ALEKS Data tab, calculate the standard reaction free energy AG° for the following redox reaction. Be sure your answer has the correct number of significant digits. 3Cu+ (aq) + Cro²¯ (aq) +4H₂O (1) → 3Cu²+ (aq) +Cr(OH)3 (s)+5OH˜¯ (aq) 0 kJ ☐ x10 00. 18 Ararrow_forward
- Calculating the pH of a weak base titrated with a strong acid An analytical chemist is titrating 241.7 mL of a 0.4900M solution of methylamine (CH3NH2) with a 0.7800M solution of HNO3. The pK of methylamine is 3.36. Calculate the pH of the base solution after the chemist has added 17.7 mL of the HNO3 solution to it. Note for advanced students: you may assume the final volume equals the initial volume of the solution plus the volume of HNO3 solution added. Round your answer to 2 decimal places. pH = ☑ ? 18 Ararrow_forwardThe following is two groups (Regular tomato sauce & Salt Reduced Tomato Sauce) of data recorded by a team analysising salt content in tomato sauce using the MOHR titration method: Regular Tomato Sauce Salt Reduced Tomato Sauce 223.4 148.7 353.7 278.2 334.6 268.7 305.6 234.4 340.0 262.7 304.3 283.2 244.7 143.6 QUESTION: For both groups of data calculate the answers attached in the image.arrow_forwardThe following is a two groups (Regular tomato sauce & Salt Reduced Tomato Sauce) of data recorded by a team analysising salt content in tomato sauce using the MOHR titration method: Regular Tomato Sauce Salt Reduced Tomato Sauce 340.0mmol/L 262.7mmol/L QUESTION: For both groups (Regular & Salt Reduced tomato sauce) of data provide answers to the following calculations below: 1. Standard Deviation (Sx) 2. T Values (t0.05,4) 3. 95% Confidence Interval (mmol/L) 4. [Na+] (mg/100 mL) 5. 95% Confidence Interval (mg/100 mL)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning


Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning
Stoichiometry - Chemistry for Massive Creatures: Crash Course Chemistry #6; Author: Crash Course;https://www.youtube.com/watch?v=UL1jmJaUkaQ;License: Standard YouTube License, CC-BY
Bonding (Ionic, Covalent & Metallic) - GCSE Chemistry; Author: Science Shorts;https://www.youtube.com/watch?v=p9MA6Od-zBA;License: Standard YouTube License, CC-BY
General Chemistry 1A. Lecture 12. Two Theories of Bonding.; Author: UCI Open;https://www.youtube.com/watch?v=dLTlL9Z1bh0;License: CC-BY