
Concept explainers
Addition of water to concentrated sulfuric acid is dangerous because it generates enough heat to boil the water, causing it to spatter out of the container. For this reason, chemists remember to add acid to water. In a dilution experiment, we calculate the amount of the more concentrated solution that must be measured out. If we place this concentrated solution (Figure 4.7) in the volumetric flask first, then dilute with water, we violate the caution “Add acid to water.” Describe a safer variation on the method shown in Figure 4.7 that allows the quantitative dilution of concentrated sulfuric acid.
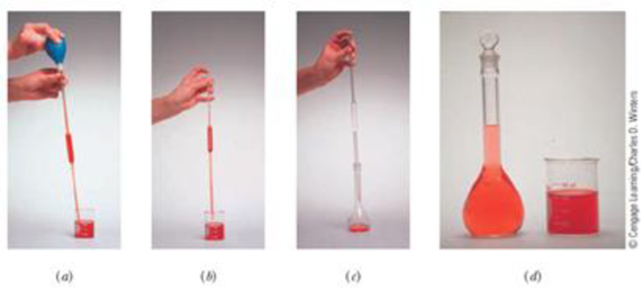
Figure 4.7 Preparing a dilute solution from a concentrated solution. (a) Draw the concentrated solution into a pipet to a level just above the calibration mark. (b) Allow the liquid to settle down to the calibration line. Touch the tip of the pipet to the side of the container to remove any extra liquid. (c) Transfer this solution to a volumetric flask. Again touch the pipet to the wall of the flask to ensure complete transfer, but do not blow out the pipet because it is calibrated for a small amount of liquid to remain in the tip. (d) Dilute to the mark with solvent. Frequently, especially with concentrated acids, it is best to have some solvent present in the volumetric flask before you add the concentrated sample.
Want to see the full answer?
Check out a sample textbook solution
Chapter 4 Solutions
Chemistry Principles And Practice
- For the condensation reaction between Alamine and histamine, please help me write the amididation reaction mechanism. Then write the three letter code for the product of the reaction, then write the one letter code for the product of the reaction. arrow_forwardHow to draw the reaction mechasnism belowarrow_forwardName the following molecules with IUpacarrow_forward
- What is the molecular orbital for cyclopropenyl anion and is it aromatic, antiaromatic or nonaromatic?arrow_forwardUsing the chart describe the change from cystine to tyrosine and its impact on the protein. Using the chart describe the change from histidine to aspartic acid and its impact on the protein.arrow_forwardHow to get the predicted product of this reaction belowarrow_forward
- Please help me fill out the chart then using the chart describe the change from cystine to tyrosine and its impact on the protein. Then using the chart describe the change from histidine to aspartic acid.arrow_forwardWrite the Esterification reaction mechanism for acetic acid, and one propanol to make propanol ethanoate (molecule that gives peas its odor in flavor)arrow_forwardProvide solutionsarrow_forward
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div
World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning





