
Chemistry Principles And Practice
3rd Edition
ISBN: 9781305295803
Author: David Reger; Scott Ball; Daniel Goode
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 4, Problem 4.3QE
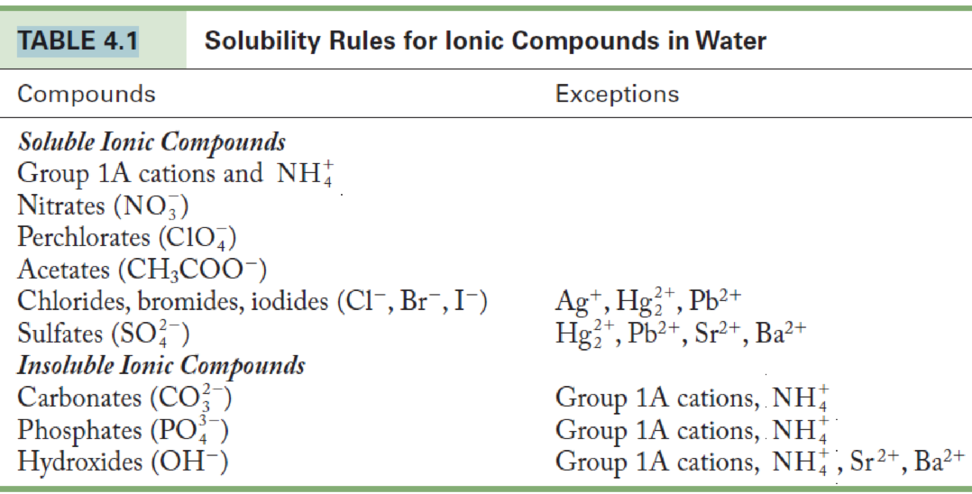
An aqueous sample is known to contain either Sr2+ or
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
For the condensation reaction between Alanine and histidine write the amididation reaction mechanism using arrows then write the three letter code for the product of the reaction and the one letter code for the product of the reaction.
Write the amididation reaction mechanism of p-aminophenol and acetic acid to produce acetaminophen please use arrows.
Name the following using IUPAC.
Chapter 4 Solutions
Chemistry Principles And Practice
Ch. 4 - Prob. 4.1QECh. 4 - A solution is formed by mixing 1 gal ethanol with...Ch. 4 - An aqueous sample is known to contain either Sr2+...Ch. 4 - Ammonium chloride is a strong electrolyte. Draw a...Ch. 4 - Experiments show that propionic acid (CH3CH2COOH)...Ch. 4 - Describe the procedure used to make 1.250 L of...Ch. 4 - If enough Li2SO4 dissolves in water to make a 0.33...Ch. 4 - Describe how 500 mL of a 1.5 M solution of HCl...Ch. 4 - Addition of water to concentrated sulfuric acid is...Ch. 4 - Draw the flow diagram for a calculation that...
Ch. 4 - Prob. 4.11QECh. 4 - Describe in words the titration of an acid with a...Ch. 4 - Describe the use of gravimetric analysis to...Ch. 4 - Draw the contents of a beaker of water that...Ch. 4 - Prob. 4.15QECh. 4 - Prob. 4.16QECh. 4 - Prob. 4.17QECh. 4 - Prob. 4.18QECh. 4 - Write the net ionic equation for the reaction, if...Ch. 4 - Write the net ionic equation for the reaction, if...Ch. 4 - Prob. 4.21QECh. 4 - Prob. 4.22QECh. 4 - Write the overall equation (including the physical...Ch. 4 - Write the overall equation (including the physical...Ch. 4 - Write the overall equation (including the physical...Ch. 4 - Write the overall equation (including the physical...Ch. 4 - An aqueous sample is known to contain either Pb2+...Ch. 4 - An aqueous sample is known to contain either Ag+...Ch. 4 - An aqueous sample is known to contain either Mg2+...Ch. 4 - An aqueous sample is known to contain either Pb2+...Ch. 4 - In the beakers shown below, the colored spheres...Ch. 4 - In the beakers shown below, the colored spheres...Ch. 4 - Calculate the molarity of KOH in a solution...Ch. 4 - Calculate the molarity of NaCl in a solution...Ch. 4 - Calculate the molarity of AgNO3 in a solution...Ch. 4 - Calculate the molarity of NaOH in a solution...Ch. 4 - What volume of a 2.3 M HCl solution is needed to...Ch. 4 - What volume of a 5.22 M NaOH solution is needed to...Ch. 4 - What volume of a 2.11 M Li2CO3 solution is needed...Ch. 4 - What volume of a 5.00 M H2SO4 solution is needed...Ch. 4 - What is the molarity of a glucose (C6H12O6)...Ch. 4 - If you dilute 25.0 mL of 1.50 M hydrochloric acid...Ch. 4 - Prob. 4.43QECh. 4 - Prob. 4.44QECh. 4 - Prob. 4.45QECh. 4 - Prob. 4.46QECh. 4 - How many grams of AgNO3 are needed to prepare 300...Ch. 4 - What mass of oxalic acid, H2C2O4, is required to...Ch. 4 - Prob. 4.49QECh. 4 - What mass of sodium sulfate, in grams, is needed...Ch. 4 - What is the molarity of a solution of strontium...Ch. 4 - What is the molarity of a solution of sodium...Ch. 4 - What is the molarity of a solution of magnesium...Ch. 4 - If 6.73 g of Na2CO3 is dissolved in enough water...Ch. 4 - The substance KSCN is frequently used to test for...Ch. 4 - Potassium permanganate (KMnO4) solutions are used...Ch. 4 - Two liters of a 1.5 M solution of sodium hydroxide...Ch. 4 - Prob. 4.58QECh. 4 - Prob. 4.59QECh. 4 - Prob. 4.60QECh. 4 - Prob. 4.61QECh. 4 - Prob. 4.62QECh. 4 - Prob. 4.63QECh. 4 - Prob. 4.64QECh. 4 - What volume of 2.4 M HCl is needed to obtain 1.3...Ch. 4 - Prob. 4.66QECh. 4 - Prob. 4.67QECh. 4 - Prob. 4.68QECh. 4 - Prob. 4.69QECh. 4 - Prob. 4.70QECh. 4 - What volume of 0.66 M HNO3 is needed to react...Ch. 4 - What volume of 0.22 M hydrochloric acid is needed...Ch. 4 - Prob. 4.73QECh. 4 - Prob. 4.74QECh. 4 - Prob. 4.75QECh. 4 - Prob. 4.76QECh. 4 - Prob. 4.77QECh. 4 - What mass of iron (III) hydroxide precipitates on...Ch. 4 - Prob. 4.79QECh. 4 - What is the solid that precipitates, and how much...Ch. 4 - What volume of 1.212 M silver nitrate is needed to...Ch. 4 - Prob. 4.82QECh. 4 - A solid forms when excess barium chloride is added...Ch. 4 - Prob. 4.84QECh. 4 - Write the overall equation (including the physical...Ch. 4 - Write the overall equation (including the physical...Ch. 4 - What is the molar concentration of a solution of...Ch. 4 - Prob. 4.88QECh. 4 - What is the molar concentration of an HCl solution...Ch. 4 - What is the molar concentration of an H2SO4...Ch. 4 - Prob. 4.91QECh. 4 - Prob. 4.92QECh. 4 - The pungent odor of vinegar is a result of the...Ch. 4 - Prob. 4.94QECh. 4 - Oranges and grapefruits are known as citrus fruits...Ch. 4 - Prob. 4.96QECh. 4 - Prob. 4.97QECh. 4 - Prob. 4.98QECh. 4 - Prob. 4.99QECh. 4 - Prob. 4.100QECh. 4 - Prob. 4.101QECh. 4 - Prob. 4.102QECh. 4 - Prob. 4.103QECh. 4 - Prob. 4.104QECh. 4 - Prob. 4.105QECh. 4 - Prob. 4.106QECh. 4 - Prob. 4.107QECh. 4 - Prob. 4.108QECh. 4 - Prob. 4.109QECh. 4 - Prob. 4.110QECh. 4 - Prob. 4.115QECh. 4 - Prob. 4.117QECh. 4 - Prob. 4.118QECh. 4 - Prob. 4.119QECh. 4 - Prob. 4.120QECh. 4 - Prob. 4.121QECh. 4 - Prob. 4.122QECh. 4 - Prob. 4.123QE
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- For the condensation reaction between Alamine and histamine, please help me write the amididation reaction mechanism. Then write the three letter code for the product of the reaction, then write the one letter code for the product of the reaction. arrow_forwardHow to draw the reaction mechasnism belowarrow_forwardName the following molecules with IUpacarrow_forward
- What is the molecular orbital for cyclopropenyl anion and is it aromatic, antiaromatic or nonaromatic?arrow_forwardUsing the chart describe the change from cystine to tyrosine and its impact on the protein. Using the chart describe the change from histidine to aspartic acid and its impact on the protein.arrow_forwardHow to get the predicted product of this reaction belowarrow_forward
- Please help me fill out the chart then using the chart describe the change from cystine to tyrosine and its impact on the protein. Then using the chart describe the change from histidine to aspartic acid.arrow_forwardWrite the Esterification reaction mechanism for acetic acid, and one propanol to make propanol ethanoate (molecule that gives peas its odor in flavor)arrow_forwardProvide solutionsarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:9781559539418
Author:Angelica Stacy
Publisher:MAC HIGHER

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning
O-Level Chemistry | 16 | Qualitative Analysis [1/3]; Author: Bernard Ng;https://www.youtube.com/watch?v=oaU8dReeBgA;License: Standard YouTube License, CC-BY