
A stream containing H2S and inert gases and a second stream of pure SO2 are fed to a sulfur recover)' reactor, where the reaction
2H2S + SO2 -» 3S + 2H2O
takes place. The feed rates are adjusted so that the ratio of H2S to SO2 in the combined feed is always stoichiometric.
In the normal operation of the reactor the flow rate and composition of the H2S feed stream both fluctuate. In the past, each time either variable changed the required SO2 feed rate had to be reset by adjusting a valve in the feed line. A control system has been installed to automate this process. The H2S feed stream passes through an electronic flowmeter that transmits a signal Rf directly proportional to the molar flow rate of the stream, h{. When = 100 kmol/h, the transmitted signal R(= 15 mV. The mole fraction of H2S in this stream is measured with a thermal conductivity detector, which transmits a signal /fa. Analyzer calibration data are as follows:
| fla(mV) | 0 | 25.4 | 42.8 | 58.0 | 71.9 | 85.1 |
| x(mol H2S/mol) | 0.00 | 0.20 | 0.40 | 0.60 | 0.80 | 1.00 |
The controller takes as input the transmitted values of Rf and R3and calculates and transmits a voltage signal j?c to a flow control valve in the SO2 line, which opens and closes to an extent dependent on the value of Rc. A plot of the SO2 flow rate. fic, versus Rcon rectangular coordinates is a straight line through the points (Rc= 10.0mV.hc= 25.0kmol/h) and (/<. = 25.0mV.hc = 60.0kmol/h).
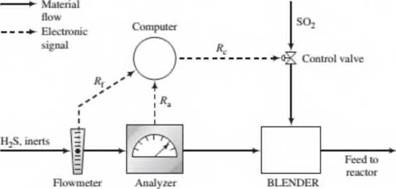
- Why would it be important to feed the reactants in stoichiometric proportion? (Hint: SO2 and especially H2S are serious pollutants.) What are several likely reasons for wanting to automate the SO2 feed rate adjustment?
- If the first stream contains 85.0 mole% H2S and enters the unit at a rate of hf = 3.00 X 102 kmol/h, what must the value of nt(kmol SO2/h) be?
- Fit a function to the H2S analyzer calibration data to derive an expression for x as a function of Rt. Check the fit by plotting both the function and the calibration data on the same graph.
- Derive a formula for Rcfrom specified values of Rf and Ra, using the result of Part (c) in the derivation. (This formula would be built into the controller.) Test the formula using the flow rate and composition data of Part (a).
- The system has been installed and made operational, and at some point the concentration of H2S in the feed stream suddenly changes. A sample of the blended gas is collected and analyzed a short time later and the mole ratio of H2S to SO2 is not the required 2:1. List as many possible reasons as you can think of for this apparent failure of the control system.
Trending nowThis is a popular solution!

Chapter 4 Solutions
ELEMENTARY PRINCIPLES OF CHEM. PROCESS.
Additional Science Textbook Solutions
Web Development and Design Foundations with HTML5 (8th Edition)
Computer Science: An Overview (13th Edition) (What's New in Computer Science)
Starting Out with Java: From Control Structures through Data Structures (4th Edition) (What's New in Computer Science)
Modern Database Management
Starting Out With Visual Basic (8th Edition)
Java How to Program, Early Objects (11th Edition) (Deitel: How to Program)
- Which of the following compounds can be synthesized using one reaction from any alkene, as a major product? If it can be synthesized, propose a route, and you may use any other starting materials, reagents and solvents as needed. If you do not think that it can be synthesized as a major product from an alkene, explain in detail why.arrow_forwardDraw the stepwise mechanism (with arrow pushing)arrow_forwarda) Explain why product 1 is the kinetic product and product 2 is the thermodynamic product. b) Draw the reaction coordinate diagram for the reaction pathway generating each product. c) State the Arrhenius Equation and explain the terms with their physical significance. d) State and explain which reaction pathway has a higher rate constant. What happens to the rate constant if the temperature has increased?arrow_forward
- Part 1. Draw monomer units of the following products and draw their reaction mechanism 1) Bakelite like polymer Using: Resorcinol + NaOH + Formalin 2) Polyester fiber Using a) pthalic anhydride + anhydrous sodium acetate + ethylene glycol B)pthalic anhydride + anhydrous sodium acetate + glycerol 3) Temporary cross-linked polymer Using: 4% polyvinyl alcohol+ methyl red + 4% sodium boratearrow_forwardUsing the table of Reactants and Products provided provide the correct letter that corresponds with the Carboxylic acid that is formed in the reaction below. 6 M NaOH Acid-workup WRITE THE CORRECT LETTER ONLY DO NOT WRITE EXTRA WORDS OR PHRASES A) Pool of Reagents for Part B CI B) OH C) E) CI J) racemic F) K) OH N) OH P) G) OH D) HO H) L) M) HO Q) R) CI Aarrow_forwardIn the table below, the exact chemical structures for Methyl salicylate can be represented by the letter WRITE THE CORRECT LETTER ONLY DO NOT WRITE EXTRA WORDS OR PHRASES CI B) A) E) Cl racemic F) J) CI K) N) OH P) Pool of Reagents for Part B OH OH G) L) OH D) HO H) M) HO Q) R) CIarrow_forward
- Draw the stepwise mechanism for the reactionsarrow_forwardPart I. a) Draw reaction mechanism for the transformations of benzophenone to benzopinacol to benzopinaco lone b) Pinacol (2,3-dimethyl, 1-3-butanediol) on treatment w/ acid gives a mixture of pina colone (3,3-dimethyl-2-butanone) and 2, 3-dimethyl - 1,3-butadiene. Give reasonable mechanism the formation of the products Forarrow_forward3. The explosive decomposition of 2 mole of TNT (2,4,6-trinitrotoluene) is shown below: Assume the C(s) is soot-basically atomic carbon (although it isn't actually atomic carbon in real life). 2 CH3 H NO2 NO2 3N2 (g)+7CO (g) + 5H₂O (g) + 7C (s) H a. Use bond dissociation energies to calculate how much AU is for this reaction in kJ/mol.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





