Problem 1E: What does it mean to say an equation is balanced? Why is it important for an equation to be... Problem 2E: Consider molecular, complete ionic, and net ionic equations. (a) What is the difference between... Problem 3E: Balance the following equations: (a) PCl5(s)+H2O(l)POCl3(l)+HCl(aq) (b) Cu(s)+HNO3(aq)Cu(... Problem 4E: Balance the following equations: (a) Ag(s)+H2S(g)+O2(g)Ag2S(s)+H2O(l) (b) P4(s)+O2(g)P4O10(s) (c)... Problem 5E: Write a balanced molecular equation describing each of the following chemical reactions. (a) Solid... Problem 6E: Write a balanced equation describing each of the following chemical reactions. (a) Solid potassium... Problem 7E: Colorful fireworks often involve the decomposition of barium nitrate and potassium chlorate and the... Problem 8E: Fill in the blank with a single chemical formula for a covalent compound that will balance the... Problem 9E: Aqueous hydrogen fluoride (hydrofluoric acid) is used to etch glass and to analyze minerals for... Problem 10E: A novel process for obtaining magnesium from sea water involves several reactions. Write a balanced... Problem 11E: From the balanced molecular equations, write the complete ionic and net ionic equations for the... Problem 12E: Use the following equations to answer the next four questions: H2O(s)H2O(l)... Problem 13E: Indicate what type, or types, of reaction each of the following represents: (a) Ca(s)+Br2(l)CaBr2(s)... Problem 14E: Indicate what type, or types, of reaction each of the following represents: (a)... Problem 15E: Silver can be separated from gold because silver dissolves in nitric acid while gold does not. Is... Problem 16E: Determine the oxidation states of the elements in the following compounds: (a) Nal (b) GdCl3 (c)... Problem 17E: Determine the oxidation states of the elements in the compounds listed. None of the... Problem 18E: Determine the oxidation states of the elements in the compounds listed. None of the... Problem 19E: Classify the following as acid-base reactions or oxidation-reduction reactions: (a)... Problem 20E: Identify the atoms that are oxidized and reduced, the change in oxidation state for each, and the... Problem 21E: Complete and balance the following acid-base equations: (a) HCl gas reacts with solid Ca(OH)2(s).... Problem 22E: Complete and balance the following acid-base equations: (a) A solution of HClO4 is added to a... Problem 23E: Complete and balance the following oxidation-reduction reactions, which give the highest possible... Problem 24E: Complete and balance the following oxidation-reduction reactions, which give the highest possible... Problem 25E: Complete and balance the equations for the following acid-base neutralization reactions. If water is... Problem 26E: When heated to 700—800 C, diamonds, which are pure carbon, are oxidized by atmospheric oxygen. (They... Problem 27E: The military has experimented with lasers that produce very intense light when fluorine combines... Problem 28E: Write the molecular, total ionic, and net ionic equations for the following reactions: (a)... Problem 29E: Great Lakes Chemical Company produces bromine, Br2, from bromide salts such as NaBr, in Arkansas... Problem 30E: In a common experiment in the general chemistry laboratory, magnesium metal is heated in air to... Problem 31E: Lithium hydroxide may be used to absorb carbon dioxide in enclosed environments, such as manned... Problem 32E: Calcium propionate is sometimes added to bread to retard spoilage. This compound can be prepared by... Problem 33E: Complete and balance the equations of the following reactions, each of which could be used to remove... Problem 34E: Copper(II) sulfide is oxidized by molecular oxygen to produce gaseous sulfur trioxide and solid... Problem 35E: Write balanced chemical equations for the reactions used to prepare each of the following compounds... Problem 36E: Calcium cyclamate Ca(C6H 11 NHSO3)2 is an artificial sweetener used in many countries around the... Problem 37E: Complete and balance each of the following half-reactions (steps 2—5 in half-reaction method): (a)... Problem 38E: Complete and balance each of the following half-reactions (steps 25 in half-reaction method): (a)... Problem 39E: Balance each of the following equations according to the half-reaction method: (a)... Problem 40E: Balance each of the following equations according to the half-reaction method: (a)... Problem 41E: Balance each of the following equations according to the half-reaction method: (a)... Problem 42E: Write the balanced equation, then outline the steps necessary to determine the information requested... Problem 43E: Write the balanced equation, then outline the steps necessary to determine the information requested... Problem 44E: Write the balanced equation, then outline the steps necessary to determine the information requested... Problem 45E: Write the balanced equation, then outline the steps necessary to determine the information requested... Problem 46E: H2 is produced by the reaction of 118.5 mL of a 0.8775-M solution of H3PO4 according to the... Problem 47E: Gallium chloride is formed by the reaction of 2.6 L of a 1.44 M solution of HCl according to the... Problem 48E: I2 is produced by the reaction of 0.4235 mol of CuCl2 according to the following equation:... Problem 49E: Silver is often extracted from ores such as K[Ag(CN)2] and then recovered by the reaction... Problem 50E: What mass of silver oxide, Ag2O, is required to produce 25.0 g of silver sulfadiazine, AgC10H9N4SO2,... Problem 51E: Carborundum is silicon carbide, SiC, a very hard material used as an abrasive on sandpaper and in... Problem 52E: Automotive air bags inflate when a sample of sodium azide, NaN3, is very rapidly decomposed.... Problem 53E: Urea, CO( NH2)2, is manufactured on a large scale for use in producing urea-formaldehyde plastics... Problem 54E: In an accident, a solution containing 2.5 kg of nitric acid was spilled. Two kilograms of Na2CO3 was... Problem 55E: A compact car gets 37.5 miles per gallon on the highway. If gasoline contains 84.2% carbon by mass... Problem 56E: What volume of 0.750 M hydrochloric acid solution can be prepared from the HCl produced by the... Problem 57E: What volume of a 0.2089 M Kl solution contains enough KI to react exactly with the Cu( NO3)2 in... Problem 58E: A mordant is a substance that combines with a dye to produce a stable fixed color in a dyed fabric.... Problem 59E: The toxic pigment called white lead, Pb3(OH)2( CO3)2, has been replaced in white paints by rutile,... Problem 60E: The following quantities are placed in a container: 1.51024 atoms of hydrogen, 1.0 mol of sulfur,... Problem 61E: What is the limiting reactant in a reaction that produces sodium chloride from 8 g of sodium and 8 g... Problem 62E: Which of the postulates of Dalton’s atomic theory explains why we can calculate a theoretical yield... Problem 63E: A student isolated 25 g of a compound following a procedure that would theoretically yield 81 g.... Problem 64E: A sample of 0.53 g of carbon dioxide was obtained by heating 1.31 g of calcium carbonate. What is... Problem 65E: Freon-12, CCl2F2, is prepared from CCl4 by reaction with HF. The other product of this reaction is... Problem 66E: Citric acid, C6H5CH3, a component of jams, jellies, and fruity soft drinks, is prepared industrially... Problem 67E: Toluene, C6H5CH3, is oxidized by air under carefully controlled conditions to benzoic acid,... Problem 68E: In a laboratory experiment, the reaction of 3.0 mol of H2 with 2.0 mol of I2 produced 1.0 mol of HI.... Problem 69E: Outline the steps needed to solve the following problem, then do the calculations. Ether, (C2H5)2O,... Problem 70E: Outline the steps needed to determine the limiting reactant when 30.0 g of propane, C3H8, is burned... Problem 71E: Outline the steps needed to determine the limiting reactant when 0.50 mol of Cr and 0.75 mol of... Problem 72E: What is the limiting reactant when 1.50 g of lithium and 1.50 g of nitrogen combine to form lithium... Problem 73E: Uranium can be isolated from its ores by dissolving it as UO2( NO3)2, then separating it as solid... Problem 74E: How many molecules of C2H4Cl2 can be prepared from 15C2H4 molecules and 8Cl2 molecules? Problem 75E: How many molecules of the sweetener saccharin can be prepared from 30 C atoms, 25 H atoms, 12 0... Problem 76E: The phosphorus pentoxide used to produce phosphoric acid for cola soft drinks is prepared by burning... Problem 77E: Would you agree to buy 1 trillion (1,000,000,000,000) gold atoms for $5? Explain why or why not.... Problem 78E: What volume of 0.0105-M HBr solution is required to titrate 125 mL of a 0.0100-M Ca(OH)2 solution?... Problem 79E: Titration of a 20.0-mL sample of acid rain required 1.7 mL of 0.08 11 M NaOH to reach the end point.... Problem 80E: What is the concentration of NaCl in a solution if titration of 15.00 mL of the solution with 0.2503... Problem 81E: In a common medical laboratory determination of the concentration of free chloride ion in blood... Problem 82E: Potatoes can be peeled commercially by soaking them in a 3-M to 6-M solution of sodium hydroxide,... Problem 83E: A sample of gallium bromide, GaBr3, weighing 0. 165 g was dissolved in water and treated with silver... Problem 84E: The principal component of mothballs is naphthalene, a compound with a molecular mass of about 130... Problem 85E: A 0.025-g sample of a compound composed of boron and hydrogen, with a molecular mass of ~28 amu,... Problem 86E: Sodium bicarbonate (baking soda), NaHCO3, can be purified by dissolving it in hot water (60 C),... Problem 87E: What volume of 0.600 M HCl is required to react completely with 2.50 g of sodium hydrogen carbonate?... Problem 88E: What volume of 0.08892 M HNO3 is required to react completely with 0.2352 g of potassium hydrogen... Problem 89E: What volume of a 0.3300-M solution of sodium hydroxide would be required to titrate 15.00 mL of... Problem 90E: What volume of a 0.00945-M solution of potassium hydroxide would be required to titrate 50.00 mL of... Problem 91E: A sample of solid calcium hydroxide, Ca(OH)2, is allowed to stand in water until a saturated... Problem 92E: What mass of Ca(OH)2 will react with 25.0 g of butanoic to form the preservative calcium butanoate... Problem 93E: How many milliliters of a 0.1500-M solution of KOH will be required to titrate 40.00 mL of a... Problem 94E: Potassium acid phthalate, KNaC8H4O4, or KHP, is used in many laboratories, including general... Problem 95E: The reaction of WCl6 with Al at ~400 C gives black crystals of a compound containing only tungsten... format_list_bulleted

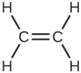 required to react with
required to react with
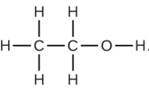

 Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning





