
Campbell Biology, Books a la Carte Plus Mastering Biology with eText -- Access Card Package (10th Edition)
10th Edition
ISBN: 9780133922851
Author: Jane B. Reece, Lisa A. Urry, Michael L. Cain, Steven A. Wasserman, Peter V. Minorsky, Robert B. Jackson
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Question
Chapter 4, Problem 2TYU
Summary Introduction
To find: The functional group not present in the Fig.1.
Pictorial representation: The molecule in the Fig.1 represents different
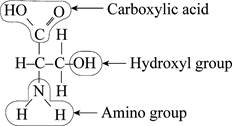
Fig.1: A molecule
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
a couple in which the father has the a blood type and the mother has the o blood type produce an offspring with the o blood type, how does this happen? how could two functionally O parents produce an offspring that has the a blood type?
What is the opening indicated by the pointer? (leaf x.s.)
stomate
guard cell
lenticel
intercellular space
none of these
Identify the indicated tissue? (stem x.s.)
parenchyma
collenchyma
sclerenchyma
○ xylem
○ phloem
none of these
Chapter 4 Solutions
Campbell Biology, Books a la Carte Plus Mastering Biology with eText -- Access Card Package (10th Edition)
Ch. 4.1 - Why was Whler astonished to find he had made urea?Ch. 4.1 - VISUAL SKILLS See Figure 4.2. Miller carried out...Ch. 4.2 - DRAW IT (a) Draw a structural formula for C2H4....Ch. 4.2 - Prob. 2CCCh. 4.2 - How are gasoline and fat chemically similar?Ch. 4.2 - VISUAL SKILLS See Figures 4.5a and 4.7. Can...Ch. 4.3 - VISUAL SKILLS What does the term amino acid...Ch. 4.3 - What chemical change occurs to ATP when it reacts...Ch. 4.3 - DRAW IT Suggose you had an organic molecule such...Ch. 4 - How did Stanley Miller's experiments support the...
Ch. 4 - Prob. 4.2CRCh. 4 - In what ways does a methyl group differ chemically...Ch. 4 - Organic chemistry is currently defined as (A) the...Ch. 4 - Prob. 2TYUCh. 4 - MAKE CONNECTIONS Which chemical group is most...Ch. 4 - VISUAL SKILLS Visualize the structural formula of...Ch. 4 - visual skills Choose the term that correctly...Ch. 4 - VISUAL SKILLS Identify the asymmetric carbon in...Ch. 4 - Which action could produce a carbonyl group? (A)...Ch. 4 - Prob. 8TYUCh. 4 - Prob. 9TYUCh. 4 - SCIENTIFIC INQUIRY 50 years ago, pregnant women...Ch. 4 - WRITE ABOUT A THEME: ORGANIZATION In 1918, an...Ch. 4 - SYNTHESIZE YOUR KNOWLEDGE Explain how the chemical...
Knowledge Booster
Similar questions
- Where did this structure originate from? (Salix branch root) epidermis cortex endodermis pericycle vascular cylinderarrow_forwardIdentify the indicated tissue. (Tilia stem x.s.) parenchyma collenchyma sclerenchyma xylem phloem none of thesearrow_forwardIdentify the indicated structure. (Cucurbita stem l.s.) pit lenticel stomate tendril none of thesearrow_forward
- Identify the specific cell? (Zebrina leaf peel) vessel element sieve element companion cell tracheid guard cell subsidiary cell none of thesearrow_forwardWhat type of cells flank the opening on either side? (leaf x.s.) vessel elements sieve elements companion cells tracheids guard cells none of thesearrow_forwardWhat specific cell is indicated. (Cucurbita stem I.s.) vessel element sieve element O companion cell tracheid guard cell none of thesearrow_forward
- Respond to the following in a minimum of 175 words: How might CRISPR-Cas 9 be used in research or, eventually, therapeutically in patients? What are some potential ethical issues associated with using this technology? Do the advantages of using this technology outweigh the disadvantages (or vice versa)? Explain your position.arrow_forwardYou are studying the effect of directional selection on body height in three populations (graphs a, b, and c below). (a) What is the selection differential? Show your calculation. (2 pts) (b) Which population has the highest narrow sense heritability for height? Explain your answer. (2 pts) (c) If you examined the offspring in the next generation in each population, which population would have the highest mean height? Why? (2 pts) (a) Midoffspring height (average height of offspring) Short Short Short Short (c) Short (b) Short Tall Short Tall Short Short Tall Midparent height (average height of Mean of population = 65 inches Mean of breading parents = 70 inches Mean of population = 65 inches Mean of breading parents = 70 inches Mean of population = 65 inches Mean of breading parents = 70 inchesarrow_forwardP You are studying a population of 100 flowers that has two alleles at a locus for flower color, blue (B) and green (G). There are 15 individuals with the BB genotype, 70 individuals with the BG genotype, and 15 individuals with the GG genotype. (a) What are the allele frequencies of B and G in the starting population? Show your calculations. (2 pts) (b) Is this population in Hardy-Weinberg equilibrium? Show your calculations. (3 pts) 12pt v Paragraph BIU UA AV & VT2V f CO Varrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Biology (MindTap Course List)BiologyISBN:9781337392938Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. BergPublisher:Cengage Learning
Biology (MindTap Course List)BiologyISBN:9781337392938Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. BergPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning Biology: The Dynamic Science (MindTap Course List)BiologyISBN:9781305389892Author:Peter J. Russell, Paul E. Hertz, Beverly McMillanPublisher:Cengage Learning
Biology: The Dynamic Science (MindTap Course List)BiologyISBN:9781305389892Author:Peter J. Russell, Paul E. Hertz, Beverly McMillanPublisher:Cengage Learning Anatomy & PhysiologyBiologyISBN:9781938168130Author:Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark WomblePublisher:OpenStax College
Anatomy & PhysiologyBiologyISBN:9781938168130Author:Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark WomblePublisher:OpenStax College Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax
Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning

Biology (MindTap Course List)
Biology
ISBN:9781337392938
Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. Berg
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biology: The Dynamic Science (MindTap Course List)
Biology
ISBN:9781305389892
Author:Peter J. Russell, Paul E. Hertz, Beverly McMillan
Publisher:Cengage Learning

Anatomy & Physiology
Biology
ISBN:9781938168130
Author:Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark Womble
Publisher:OpenStax College

Biology 2e
Biology
ISBN:9781947172517
Author:Matthew Douglas, Jung Choi, Mary Ann Clark
Publisher:OpenStax

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning