
Organic Chemistry As a Second Language: Second Semester Topics
4th Edition
ISBN: 9781119110651
Author: David R. Klein
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 3.5, Problem 3.33P
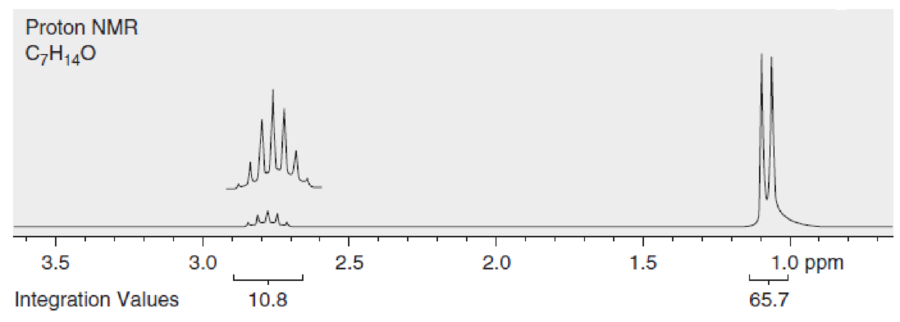
Below are NMR spectra of several compounds. Identify whether these compounds are likely to contain ethyl, isopropyl, and/or tert-butyl groups:
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Blocking Group are use to put 2 large sterically repulsive group ortho. Show the correct sequence toconnect the reagent to product with the highest yield possible. * see image **NOTE: The compound on the left is the starting point, and the compound on the right is the final product. Please show the steps in between to get from start to final, please. These are not two different compounds that need to be worked.
I dont understand this.
Can you please explain this prooblem to me, show me how the conjugation is added, did I add them in the correct places and if so please show me. Thanks!
Chapter 3 Solutions
Organic Chemistry As a Second Language: Second Semester Topics
Ch. 3.1 - Prob. 3.2PCh. 3.1 - Prob. 3.3PCh. 3.1 - Prob. 3.4PCh. 3.1 - Prob. 3.5PCh. 3.1 - Prob. 3.6PCh. 3.1 - Prob. 3.7PCh. 3.1 - Prob. 3.8PCh. 3.1 - Prob. 3.9PCh. 3.1 - Prob. 3.10PCh. 3.1 - If you look at your answers to the previous...
Ch. 3.2 - Predict the chemical shifts for the signals in the...Ch. 3.2 - Predict the chemical shifts for the signals in the...Ch. 3.2 - Predict the chemical shifts for the signals in the...Ch. 3.2 - Predict the chemical shifts for the signals in the...Ch. 3.2 - Predict the chemical shifts for the signals in the...Ch. 3.2 - Predict the chemical shifts for the signals in the...Ch. 3.2 - Prob. 3.19PCh. 3.3 - Prob. 3.21PCh. 3.3 - Prob. 3.22PCh. 3.3 - Prob. 3.23PCh. 3.4 - Predict the multiplicity of each signal in the...Ch. 3.4 - Predict the multiplicity of each signal in the...Ch. 3.4 - Predict the multiplicity of each signal in the...Ch. 3.4 - Predict the multiplicity of each signal in the...Ch. 3.4 - Predict the multiplicity of each signal in the...Ch. 3.4 - Predict the multiplicity of each signal in the...Ch. 3.5 - Below are NMR spectra of several compounds....Ch. 3.5 - Below are NMR spectra of several compounds....Ch. 3.5 - Below are NMR spectra of several compounds....Ch. 3.8 - Calculate the degree of unsaturation for each of...Ch. 3.8 - Calculate the degree of unsaturation for each of...Ch. 3.8 - Calculate the degree of unsaturation for each of...Ch. 3.8 - Calculate the degree of unsaturation for each of...Ch. 3.8 - Calculate the degree of unsaturation for each of...Ch. 3.8 - Calculate the degree of unsaturation for each of...Ch. 3.9 - Prob. 3.43PCh. 3.9 - Propose a structure for a compound with molecular...Ch. 3.9 - Propose a structure for a compound with molecular...Ch. 3.9 - Propose a structure for a compound with molecular...Ch. 3.9 - Propose a structure for a compound with molecular...Ch. 3.9 - Prob. 3.48PCh. 3.10 - For each compound below, predict the number of...Ch. 3.10 - For each compound below, predict the number of...Ch. 3.10 - For each compound below, predict the number of...Ch. 3.10 - For each compound below, predict the number of...Ch. 3.10 - For each compound below, predict the number of...Ch. 3.10 - For each compound below, predict the number of...
Additional Science Textbook Solutions
Find more solutions based on key concepts
Some organizations are starting to envision a sustainable societyone in which each generation inherits sufficie...
Campbell Essential Biology (7th Edition)
What properties do all types of epithelia share?
Campbell Biology (11th Edition)
1.6 Read the labels on products used to wash your dishes. What are the names of some chemicals contained in tho...
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
Flask A contains yeast cells in glucose-minimal salts broth incubated at 30C with aeration. Flask B contains ye...
Microbiology: An Introduction
What two body structures contain flexible elastic cartilage?
Anatomy & Physiology (6th Edition)
Modified True/False 9. A giant bacterium that is large enough to be seen without a microscope is Selenomonas.
Microbiology with Diseases by Body System (5th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Basic strength of organic bases.arrow_forwardNucleophilic Aromatic Substitution: What is the product of the reaction? What is the name of the intermediate complex? *See imagearrow_forwardPredict the final product. If 2 products are made, list which should be “major” and “minor” *see attachedarrow_forward
- Nucleophilic Aromatic Substitution: What is the product of the reaction? *see imagearrow_forwardShow the correct sequence to connect the reagent to product. * see imagearrow_forwardThe answer here says that F and K have a singlet and a doublet. The singlet and doublet are referring to the H's 1 carbon away from the carbon attached to the OH. Why don't the H's two carbons away, the ones on the cyclohexane ring, cause more peaks on the signal?arrow_forward
- Draw the Birch Reduction for this aromatic compound and include electron withdrawing groups and electron donating groups. *See attachedarrow_forwardShow the correct sequence to connect the reagent to product. * see imagearrow_forwardBlocking Group are use to put 2 large sterically repulsive group ortho. Show the correct sequence toconnect the reagent to product with the highest yield possible. * see imagearrow_forward
- Elimination-Addition: What molecule was determined to be an intermediate based on a “trapping experiment”? *please solve and see imagearrow_forwardShow the correct sequence to connect the reagent to product. * see imagearrow_forwardPredict the final product. If 2 products are made, list which should be “major” and “minor”. **see attachedarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
NMR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=SBir5wUS3Bo;License: Standard YouTube License, CC-BY