
Organic Chemistry As a Second Language: Second Semester Topics
4th Edition
ISBN: 9781119110651
Author: David R. Klein
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 3.10, Problem 3.53P
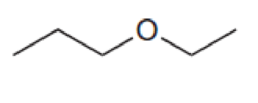
For each compound below, predict the number of signals and the location of each signal in the expected 13C NMR spectrum.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Determine the multiplicity of each signal in the expected 1H and 13C NMR spectrum of the following compound.
Can i get help with this problem
Which compound gives a signal in the 1H-NMR spectrum with a larger chemical shift, furan or cyclopentadiene? Explain.
Chapter 3 Solutions
Organic Chemistry As a Second Language: Second Semester Topics
Ch. 3.1 - Prob. 3.2PCh. 3.1 - Prob. 3.3PCh. 3.1 - Prob. 3.4PCh. 3.1 - Prob. 3.5PCh. 3.1 - Prob. 3.6PCh. 3.1 - Prob. 3.7PCh. 3.1 - Prob. 3.8PCh. 3.1 - Prob. 3.9PCh. 3.1 - Prob. 3.10PCh. 3.1 - If you look at your answers to the previous...
Ch. 3.2 - Predict the chemical shifts for the signals in the...Ch. 3.2 - Predict the chemical shifts for the signals in the...Ch. 3.2 - Predict the chemical shifts for the signals in the...Ch. 3.2 - Predict the chemical shifts for the signals in the...Ch. 3.2 - Predict the chemical shifts for the signals in the...Ch. 3.2 - Predict the chemical shifts for the signals in the...Ch. 3.2 - Prob. 3.19PCh. 3.3 - Prob. 3.21PCh. 3.3 - Prob. 3.22PCh. 3.3 - Prob. 3.23PCh. 3.4 - Predict the multiplicity of each signal in the...Ch. 3.4 - Predict the multiplicity of each signal in the...Ch. 3.4 - Predict the multiplicity of each signal in the...Ch. 3.4 - Predict the multiplicity of each signal in the...Ch. 3.4 - Predict the multiplicity of each signal in the...Ch. 3.4 - Predict the multiplicity of each signal in the...Ch. 3.5 - Below are NMR spectra of several compounds....Ch. 3.5 - Below are NMR spectra of several compounds....Ch. 3.5 - Below are NMR spectra of several compounds....Ch. 3.8 - Calculate the degree of unsaturation for each of...Ch. 3.8 - Calculate the degree of unsaturation for each of...Ch. 3.8 - Calculate the degree of unsaturation for each of...Ch. 3.8 - Calculate the degree of unsaturation for each of...Ch. 3.8 - Calculate the degree of unsaturation for each of...Ch. 3.8 - Calculate the degree of unsaturation for each of...Ch. 3.9 - Prob. 3.43PCh. 3.9 - Propose a structure for a compound with molecular...Ch. 3.9 - Propose a structure for a compound with molecular...Ch. 3.9 - Propose a structure for a compound with molecular...Ch. 3.9 - Propose a structure for a compound with molecular...Ch. 3.9 - Prob. 3.48PCh. 3.10 - For each compound below, predict the number of...Ch. 3.10 - For each compound below, predict the number of...Ch. 3.10 - For each compound below, predict the number of...Ch. 3.10 - For each compound below, predict the number of...Ch. 3.10 - For each compound below, predict the number of...Ch. 3.10 - For each compound below, predict the number of...
Additional Science Textbook Solutions
Find more solutions based on key concepts
Predict whether a precipitation reaction will occur when aqueous solutions of the following substances are mixe...
CHEMISTRY-TEXT
An atom with a formal charge does not necessarily have more or less electron density than the atoms in the mole...
Organic Chemistry (8th Edition)
9.1 Calculate the total mass of the reactants and the products for each of the following equations:
Basic Chemistry (5th Edition)
39. Determine the molecular geometry and sketch each molecule or ion using the bond conventions shown in "Repre...
Chemistry: A Molecular Approach
The IR and 1H NMR spectra for compound X(C8H10) are given in Fig. 14.31. Propose a structure for compound X.
Organic Chemistry
2.81 In which of the fo1losing pairs do both numbers contain the same number of significant figures? (2.2)
a....
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Following are two constitutional isomers with the molecular formula C4H8O2. (a) Predict the number of signals in the 1H-NMR spectrum of each isomer. (b) Predict the ratio of areas of the signals in each spectrum. (c) Show how you can distinguish between these isomers on the basis of chemical shift.arrow_forwardThe following 1H NMR peaks were recorded on a spectrometer operating at 200 MHz. Convert each into δ units. (a) CHCl3; 1454 Hz (b) CH3Cl; 610 Hz (c) CH3OH; 693 Hz (d) CH2Cl2; 1060 Hzarrow_forwardFollowing are three compounds with the molecular formula C4H8O2 and three 1H-NMR spectra. Assign each compound its correct spectrum and assign all signals to their corresponding hydrogens. (1) (2) (3)arrow_forward
- Propose structures for compounds that fit the following descriptions: (a) A hydrocarbon with seven lines in its 13C NMR spectrum (b) A six-carbon compound with only five lines in its 13C NMR spectrum (c) A four-carbon compound with three lines in its 13C NMR spectrumarrow_forwardPredict the number of signals and the splitting pattern of each signal in the 1H-NMR spectrum of each moleculearrow_forwardPredict the number of signals in a proton-decoupled 13C-NMR spectrum of each compoundarrow_forward
- How many signals are present in the 1H NMR spectrum for each molecule? What splitting is observed in each signal?arrow_forwardPredict the number of signals in a proton-decoupled 13C-NMR spectrum of each compoundarrow_forwardhow to determine the multiplicity of each signal in the expected 1H NMR spectrumarrow_forward
- Predict the chemical shift and spiting pattern of each of the signals in the 1H NMR spectrum and sketch the spectrum of compound A above.arrow_forwardCan you please confirm if this H-NMR spectrum belongs to this molecule and identify the signals of each spectrum.arrow_forwardThe 1H-NMR spectrum of 1-chloropropane shows three signals and the 1H-NMR spectrum of 2-chloropropane shows two signals. Draw these two molecules and determine the relative integrals of each signal.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

NMR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=SBir5wUS3Bo;License: Standard YouTube License, CC-BY