
Organic Chemistry As a Second Language: Second Semester Topics
4th Edition
ISBN: 9781119110651
Author: David R. Klein
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 3.9, Problem 3.46P
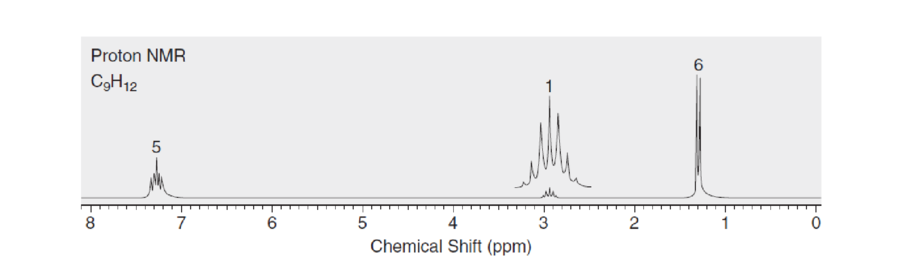
Propose a structure for a compound with molecular formula C9H12 that is consistent with the following proton NMR spectrum.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Pls explain too
Determine the structure of the compound C6H100
whose 'H NMR spectrum is shown here.
C6H100
6.5
25
7
6
3
1
Chemical shift (ppm)
6) Draw the 13C NMR spectrum for the following compound.
Chapter 3 Solutions
Organic Chemistry As a Second Language: Second Semester Topics
Ch. 3.1 - Prob. 3.2PCh. 3.1 - Prob. 3.3PCh. 3.1 - Prob. 3.4PCh. 3.1 - Prob. 3.5PCh. 3.1 - Prob. 3.6PCh. 3.1 - Prob. 3.7PCh. 3.1 - Prob. 3.8PCh. 3.1 - Prob. 3.9PCh. 3.1 - Prob. 3.10PCh. 3.1 - If you look at your answers to the previous...
Ch. 3.2 - Predict the chemical shifts for the signals in the...Ch. 3.2 - Predict the chemical shifts for the signals in the...Ch. 3.2 - Predict the chemical shifts for the signals in the...Ch. 3.2 - Predict the chemical shifts for the signals in the...Ch. 3.2 - Predict the chemical shifts for the signals in the...Ch. 3.2 - Predict the chemical shifts for the signals in the...Ch. 3.2 - Prob. 3.19PCh. 3.3 - Prob. 3.21PCh. 3.3 - Prob. 3.22PCh. 3.3 - Prob. 3.23PCh. 3.4 - Predict the multiplicity of each signal in the...Ch. 3.4 - Predict the multiplicity of each signal in the...Ch. 3.4 - Predict the multiplicity of each signal in the...Ch. 3.4 - Predict the multiplicity of each signal in the...Ch. 3.4 - Predict the multiplicity of each signal in the...Ch. 3.4 - Predict the multiplicity of each signal in the...Ch. 3.5 - Below are NMR spectra of several compounds....Ch. 3.5 - Below are NMR spectra of several compounds....Ch. 3.5 - Below are NMR spectra of several compounds....Ch. 3.8 - Calculate the degree of unsaturation for each of...Ch. 3.8 - Calculate the degree of unsaturation for each of...Ch. 3.8 - Calculate the degree of unsaturation for each of...Ch. 3.8 - Calculate the degree of unsaturation for each of...Ch. 3.8 - Calculate the degree of unsaturation for each of...Ch. 3.8 - Calculate the degree of unsaturation for each of...Ch. 3.9 - Prob. 3.43PCh. 3.9 - Propose a structure for a compound with molecular...Ch. 3.9 - Propose a structure for a compound with molecular...Ch. 3.9 - Propose a structure for a compound with molecular...Ch. 3.9 - Propose a structure for a compound with molecular...Ch. 3.9 - Prob. 3.48PCh. 3.10 - For each compound below, predict the number of...Ch. 3.10 - For each compound below, predict the number of...Ch. 3.10 - For each compound below, predict the number of...Ch. 3.10 - For each compound below, predict the number of...Ch. 3.10 - For each compound below, predict the number of...Ch. 3.10 - For each compound below, predict the number of...
Additional Science Textbook Solutions
Find more solutions based on key concepts
6.1 State the number of electrons that be must be lost by atoms of each of the following to achieve a stable el...
Chemistry: An Introduction to General, Organic, and Biological Chemistry (12th Edition) - Standalone book
Out of the given graph, that shows the correct graph for the activation energy of an endothermic reaction Conce...
Chemistry: Matter and Change
45. The tabulated data were collected for this reaction:
[NO2](M) [F2](M) Initial Rate (M/s)
0.100 0.100...
Chemistry: Structure and Properties (2nd Edition)
9.1 Calculate the total mass of the reactants and the products for each of the following equations:
Basic Chemistry (5th Edition)
Air is heated from 25°C to 140°C prior to entering a combustion furnace. The change in specific enthalpy associ...
Elementary Principles of Chemical Processes, Binder Ready Version
The following reaction has a value of G = 2.1kJ/mol(0.50kcaI/mol). CH3Br + H2S CH3 SH + HBr a. Calculate Keq a...
Organic Chemistry (9th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Below are the ¹H NMR spectrum of triphenylmethanol, benzophenone, and bromobenzene. Identify the compound corresponding to each ¹H NMR spectrum and draw the structure next to the ¹H NMR spectrum. Assign ALL peaks in each of the three ¹H NMR spectra. Hint: Conjugated systems (benzophenone) including an electronegative atom will cause a more downfield shift of ring protons in ¹H NMR compared with non-conjugated systems (bromobenzene). 8 8 8 7 7 7 6 6 6 5 5 5 4 PPM 4 PPM 4 PPM 3 3 3 2 2 2 1 1 1 0 0 0arrow_forwardPredict the 1H NMR spectrum for the compound below and sketch it. Include splitting, and label the protons and their corresponding peaks.arrow_forwardAn organic compound with molecular formula C4H8O2 has the following nmr values. Propose the structure of the compound? Shift (ppm) Area 4.13 2.05 quartet 2.02 3.04 singlet 1.15 3.00 tripletarrow_forward
- An NMR spectrum was run for the following compound. Assign proton NMR peaks and explain.arrow_forward(a) Rank each proton shown below in terms of relative chemical shift in the ¹H NMR spectrum. (1 = most upfield, 3 = most downfield) (i) (iii) H JO [ Ⓒarrow_forwardEthyl benzoate PhCO₂Et has these peaks in its 13C NMR spectrum: 17.3, 61.1, 100- 150 (four peaks) and 166.8 ppm. Which peak belongs to which carbon atom? Draw the structure of ethyl benzoate and clearly identify each carbon with its corresponding chemical shift.arrow_forward
- Following are the 'H and 13C NMR spectra for each of three isomeric ketones with formula C7H14O. Determine a structure to each pair of spectra and assign each H and C. Carbon spectrum А C,H140 Carbon spectrum В C;H140 CDCI3 200 150 100 50 Proton spectrum CDC13 A C,H140 200 150 100 50 1.96 2.00 2.91 3.0 2.5 2.0 1.5 1.0 0.5 0.0 211.04 -44.79 –17.39 – 13.78 -218.40 - 38.85 –18.55arrow_forwardHow would the 1H NMR spectra for the four compounds with molecular formula C3H6Br2 differ?arrow_forwardHow many unique 1H NMR signals are present in the following molecule?arrow_forward
- What is the structure of the compound in the following ¹H-NMR spectrum with the molecular formula C9H10O₂? Relative integration is shown. HO₂C CO₂H CO₂H CHO CO₂H PPM 2arrow_forwardHow many distinct signals would appear in the (proton-decoupled) 13C NMR spectrum for the following compound? 7 8 9arrow_forwardWhat is the structure of an unknown compound with molecular formula C6H15N that gives the following 1H NMR absorptions: 0.9 (singlet, 1 H), 1.10 (triplet, 3 H), 1.15 (singlet, 9 H), and 2.6 (quartet, 2 H) ppm?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
 EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning


EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:9781305446021
Author:Lampman
Publisher:CENGAGE LEARNING - CONSIGNMENT
NMR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=SBir5wUS3Bo;License: Standard YouTube License, CC-BY