
Interpretation:
Predict the result of the catalytic hydrogention of natural rubber and will the product obtained show syndiotactic, atactic or isotactic behaviour.
Concept introduction:
Isotactic, syndiotactic, and atactic are the stereochemical forms. The
Syndiotactic are the macromolecules in which the (-R) groups are arranged in an alternate manner along the long chain of the polymer. Gutta-percha is also an example for Syndiotactic polymer.
In atactic form, the substituents are placed in a random manner along the long chain.
The important point to note here is that the polymer obtained from the chain-growth
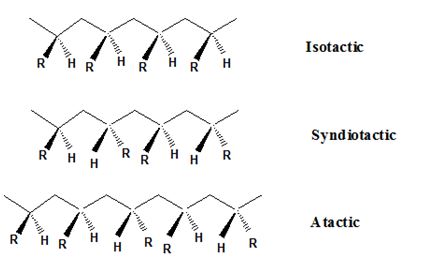
The catalytic hydrogenation natural rubber requires the breaking of double bond and the addition of hydrogen. The double bond of
Therefore, it breaks and hydrogen adds to it. When the hydrogen adds, then the double bonds are replaced with the single bonds. The hydrogenation of double bond releases energy therefore, known as an exothermic reaction. The heat released is called the heat of hydrogenation.
The catalyst used for the purpose of hydrogenation can be Ra-Ni (Raney-Nickel), PtO2 (Platinum oxide), Pd-C (Palladium on carbon) etc. They can be used to enhance the
Trending nowThis is a popular solution!

Chapter 31 Solutions
EBK ORGANIC CHEMISTRY
- Provide the unknown for the given data.arrow_forwardDraw the Lewis structures of two methanol (CH3OH) molecules and depict hydrogenbonding between them with dashed lines. Show all lone pairs. Provide a thorough analysis to apply concept idea into other problems.arrow_forwardSteps and explanation please.arrow_forward
- How could you distinguish between each pair of compounds below using IR? For each pair citeone bond and it’s frequency that you could use to distinguish between them. Please provide thorough analysis to apply into further problems.arrow_forwardSteps and explanation please.arrow_forwardSteps and explanation on how to solve.arrow_forward
- Provide the unknown for the given data.arrow_forwardElectron Arrangement A. Fill in the following chart relating to levels, sublevels and orbitals. Levels (n) 1 Sublevels # of Orbitals per sublevel 2 3 4 # of Electrons per sublevel Total Electrons per level Complete: B. Answer the following questions related to levels, sublevels, orbitals and electrons. 1. How many sublevels are in energy level 2? 2. How many orbitals are in a 4f sublevel? 3. How many electrons can level 3 hold? 4. How many orbitals are in level 4? 5. How many electrons can sublevel 2p hold? 11arrow_forwardProvide the unknown for the given details.arrow_forward
 Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning



