
EBK ORGANIC CHEMISTRY
8th Edition
ISBN: 8220102744127
Author: Bruice
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 3.1, Problem 3P
Name each of the following:
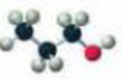
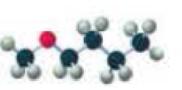
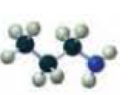
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
HCI
Draw the major products of the
following reaction:
HCI
For each molecule, assign each stereocenter as R or S. Circle the meso compounds. Label each compound as chiral or achiral.
Chapter 3 Solutions
EBK ORGANIC CHEMISTRY
Ch. 3.1 - Name each of the following:Ch. 3.1 - Draw the structure of a compound with molecular...Ch. 3.1 - Draw the structures and name the four...Ch. 3.1 - Prob. 6PCh. 3.1 - Draw the structure for each of the following: a....Ch. 3.1 - Name the following compounds: a. CH3OCH2CH3 b....Ch. 3.2 - Prob. 9PCh. 3.2 - Draw the structure for each of the following: a....Ch. 3.2 - Give each substituent on the nine-carbon chain a...Ch. 3.2 - Prob. 14P
Ch. 3.3 - What is each compounds systematic name?Ch. 3.3 - Prob. 16PCh. 3.3 - Prob. 17PCh. 3.3 - Prob. 18PCh. 3.3 - Prob. 19PCh. 3.4 - Give two names for each of the following alkyl...Ch. 3.4 - Prob. 21PCh. 3.5 - a. What is each ethers systematic name? 1....Ch. 3.6 - Give each of the following a systematic name and...Ch. 3.6 - Draw the structures of a homologous series of...Ch. 3.6 - Prob. 25PCh. 3.6 - Prob. 26PCh. 3.7 - Prob. 27PCh. 3.7 - Are the following compounds primary, secondary, or...Ch. 3.7 - Draw condensed and skeletal structures for each of...Ch. 3.7 - For each of the following, give the systematic...Ch. 3.8 - Predict the approximate size of the following bond...Ch. 3.9 - Prob. 32PCh. 3.9 - Prob. 33PCh. 3.9 - Prob. 34PCh. 3.9 - Rank the following compounds from highest boiling...Ch. 3.9 - Rank the compounds in each set from highest...Ch. 3.10 - In which solvent would cyclohexane have the lowest...Ch. 3.10 - Prob. 38PCh. 3.10 - Prob. 39PCh. 3.11 - a. Draw all the staggered and eclipsed conformers...Ch. 3.11 - Prob. 41PCh. 3.11 - Using Newman projections, draw the most stable...Ch. 3.12 - The bond angles in a regular polygon with n sides...Ch. 3.12 - Prob. 44PCh. 3.13 - Draw 1,2,3,4,5,6-hexachlorocydohexane with a. all...Ch. 3.14 - Using the data in Table 3.9, calculate the...Ch. 3.14 - The chair conformer of fluorocyclohexane is 0.25...Ch. 3.15 - Prob. 48PCh. 3.15 - Which has a higher percentage of the...Ch. 3.15 - a. Draw the more stable chair conformer of...Ch. 3.15 - For each of the following disubstituted...Ch. 3.15 - a. Draw Newman projections of the two conformers...Ch. 3.15 - a. Calculate the energy difference between the two...Ch. 3 - a. How many hydrogen does an alkene with 17...Ch. 3 - Draw the structure of octane and isooctaneCh. 3 - Draw a condensed structure and a skeletal...Ch. 3 - Prob. 56PCh. 3 - a. What is each compounds systematic name? b. Draw...Ch. 3 - Which of the following represents a cis isomer?Ch. 3 - a. How many primary carbons does each of the...Ch. 3 - Which of the following conformers of isobutyl...Ch. 3 - Draw a skeletal structure for an alkane that has...Ch. 3 - What is each compounds systematic name? a....Ch. 3 - Which bus a. the higher boiling point:...Ch. 3 - a. Draw Newman projections of the two conformers...Ch. 3 - Ansaid and Motrin belong to the group of drugs...Ch. 3 - Prob. 66PCh. 3 - A student was given the structural formulas of...Ch. 3 - Which of the following conformers has the highest...Ch. 3 - Prob. 69PCh. 3 - Draw skeletal structures for the following: a....Ch. 3 - For rotation about the C-3C-4 bond of...Ch. 3 - Prob. 72PCh. 3 - What is each compounds systematic name? a. b. c....Ch. 3 - Draw the two chair conformers for each of the...Ch. 3 - Why are lower molecular weight alcohols more...Ch. 3 - a. Draw a potential energy diagram for rotation...Ch. 3 - For each of the following compound, determine...Ch. 3 - How many ethers have molecular formula C5H12O?...Ch. 3 - Draw the most stable conformer of the following...Ch. 3 - What is each compounds systematic name?Ch. 3 - Calculate the energy difference between the two...Ch. 3 - The most stable from of glucose (blood sugar) is a...Ch. 3 - What is each compound s systematic name?Ch. 3 - Explain the following: a. 1-Hexanol has a higher...Ch. 3 - One of the chair conformers of cis-...Ch. 3 - Bromine is a larger atom than chlorine, but the...Ch. 3 - Name the following compounds:Ch. 3 - Prob. 88PCh. 3 - Using the data obtained in Problem 85, calculate...Ch. 3 - Draw the conformers for the following...
Additional Science Textbook Solutions
Find more solutions based on key concepts
73. Write a formula for each molecular compound
a. carbon monoxide
b. disulfulr tetrafiuoride
C.dichlorine m...
Introductory Chemistry (6th Edition)
Why are BSL-4 suits pressurized? Why not just wear tough regular suits?
Microbiology with Diseases by Body System (5th Edition)
The distances you obtained in Question 3 are for only one side of the ridge. Assuming that a ridge spreads equa...
Applications and Investigations in Earth Science (9th Edition)
EVOLUTION CONNECTION Crossing over is thought to be evolutionarily advantageous because it continually shuffles...
Campbell Biology (11th Edition)
Which one of the following is not a fuel produced by microorganisms? a. algal oil b. ethanol c. hydrogen d. met...
Microbiology: An Introduction
4. How do gross anatomy and microscopic anatomy differ?
Human Anatomy & Physiology (2nd Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Blackboard app.aktiv.com X Organic Chemistry II Lecture (mx Aktiv Learning App Curved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. Problem 25 of 35 Select to Edit Arrows CH3CH2OK, CH3CH2OH L Gemini M 31 0:0 :0: 5x Undo Reset Done :0: Harrow_forwardI have some reactions here for which I need to predict the products. Can you help me solve them and rewrite the equations, as well as identify the type of reaction? Please explain it to me.I have some reactions here for which I need to predict the products. Can you help me solve them and rewrite the equations, as well as identify the type of reaction? Please explain it to marrow_forwardDraw the major product of this reaction. Ignore inorganic byproducts. Problem 17 of 35 1. CH3CH2Li O H 2. Neutralizing work-up @ Atoms, Bonds and Rings Draw or tap a new boarrow_forward
- Will this convert the C=O to an alcohol? Or does its participation in the carboxy group prevent that from happening?arrow_forwardI have some reactions here for which I need to predict the products. Can you help me solve them and rewrite the equations, as well as identify the type of reaction? Please explain it to me.I have some reactions here for which I need to predict the products. Can you help me solve them and rewrite the equations, as well as identify the type of reaction? Please explain it to marrow_forwardDon't used hand raiting and don't used Ai solutionarrow_forward
- Can you explain how to draw a molecular orbital diagram for the given molecule? It is quite difficult to understand. Additionally, could you provide a clearer illustration? Furthermore, please explain how to draw molecular orbital diagrams for any other given molecule or compound as well.arrow_forwardCurved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. Prob 10: Select to Add Arrows THEarrow_forwardCurved arrows are used to illustrate the flow of electrons using the provided starting and product structures draw the curved electron pushing arrows for the following reaction or mechanistic steps Ether(solvent)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning


Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning


Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning
Nomenclature: Crash Course Chemistry #44; Author: CrashCourse;https://www.youtube.com/watch?v=U7wavimfNFE;License: Standard YouTube License, CC-BY