
Concept explainers
Arrange the following pairs of charged particles in order of increasing magnitude of electrostatic attraction (Eel).
(a) i < ii < iii < iv
(b) iv < iii < ii < i
(c) i = iii < ii < iv
(d) ii < i = iii < iv
(e) iv < i = ii < iii
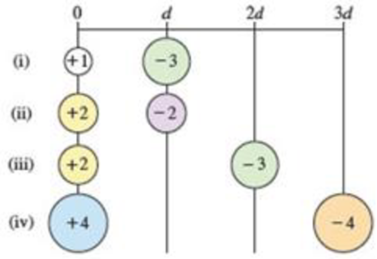
Interpretation:
The electrostatic potential energies between the given charges in the given statement should be compared by using the concept of electrostatic energy.
Concept Introduction:
Energy is the ability to do work or transfer heat where work is the movement of a body using some force. The SI unit of energy is joule (
Electrostatic energy is potential energy which results from the interaction of charged particles. Oppositely charged particles attract each other and particles of like charges repel each other. The magnitude of the resulting electrostatic potential energy is proportional to the product of the two charges (
If the charges
To calculate: Determine the
Answer to Problem 3.1.3SR
The given pairs of charged particles in order of increasing magnitude of electrostatic attraction (
Explanation of Solution
Reason for correct option
The electrostatic energy between two charges is calculated using the formulae:
It is used to compare the magnitudes of the
For charges +1 and −3:
For charges +2 and −2:
For charges +2 and −3:
For charges +4 and −4:
Therefore, the given pairs of charged particles in order of increasing magnitude of electrostatic attraction (
Reasons for incorrect options:
By doing the calculations in which the given values are substituted, it is found that the option (a) is correct. Therefore, the options (b), (c), (d) and (e) are incorrect.
The electrostatic potential energies between the given charges in the given statement are compared by using the concept of electrostatic energy.
Want to see more full solutions like this?
Chapter 3 Solutions
GEN COMBO CHEMISTRY: ATOMS FIRST; ALEKS 360 2S ACCESS CARD CHEMISTRY:ATOMS FIRST
- 2. 200 LOD For an unknown compound with a molecular ion of 101 m/z: a. Use the molecular ion to propose at least two molecular formulas. (show your work) b. What is the DU for each of your possible formulas? (show your work) C. Solve the structure and assign each of the following spectra. 8 6 4 2 (ppm) 150 100 50 ō (ppm) 4000 3000 2000 1500 1000 500 HAVENUMBERI-11arrow_forwardComplete the spectroscopy with structurearrow_forwardComplete the spectroscopy with structurearrow_forward
- Given the following concentrations for a system, calculate the value for the reaction quotient: Cl2(g)+ CS2(g) ⇌ CCl4(g)+ S2Cl2(g) Cl2 = 31.1 atm CS2 = 91.2 atm CCl4 = 2.12 atm S2Cl2 = 10.4 atmarrow_forwardMatch each chemical or item with the proper disposal or cleanup mwthod, Not all disposal and cleanup methods will be labeled. Metal sheets C, calcium, choroide solutions part A, damp metal pieces Part B, volumetric flask part A. a.Return to correct lables”drying out breaker. Place used items in the drawer.: Rinse with deionized water, dry as best you can, return to instructor. Return used material to the instructor.: Pour down the sink with planty of running water.: f.Pour into aqueous waste container. g.Places used items in garbage.arrow_forwardWrite the equilibrium constant expression for the following reaction: HNO2(aq) + H2O(l) ⇌ H3O+(aq) + NO2-(aq)arrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning



