
ORGANIC CHEMISTRY
5th Edition
ISBN: 9781259977596
Author: SMITH
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 31, Problem 31.29P
An isoprene unit can be thought of as having a head and a tail. The “head” of the isoprene unit is located at the end of the chain nearest the branch point, and the “tail” is located at the end of the carbon chain farthest from the branch point. Most isoprene units are connected together in a “head-to-tail” fashion, as illustrated. For both lycopene (Problem 31.26), and squalene (Figure 31.9), decide which isoprene units are connected in a head-to-tail fashion and which are not.
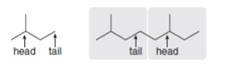
These two isoprene units are
connected in head-tail fashion.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
x +
LEKS: Using a phase diagram a X
n/alekscgi/x/lsl.exe/10_u-IgNsikr7j8P3jH-IQs_pBan HhvlTCeeBZbufu BYTI0Hz7m7D3ZcHYUt80XL-5alyVpw
○ States of Matter
Using a phase diagram to find a phase transition temperature or pressure
Use the phase diagram of Substance X below to find the melting point of X when the pressure above the solid is 1.1 atm.
pressure (atm)
16
08-
solid
liquid-
0
200
400
gas
600
temperature (K)
Note: your answer must be within 25 °C of the exact answer to be graded correct.
×
5
S: Using a phase diagram
leksogi/x/sl.exe/1ou-IgNs kr 7j8P3jH-IQs_pBan HhvTCeeBZbufuBYTI0Hz7m7D3ZdHYU+80XL-5alyVp
O States of Matter
Using a phase diagram to find a phase transition temperature or pressure
se the phase diagram of Substance X below to find the boiling point of X when the pressure on the liquid is 1.6 atm.
pressure (atm)
32-
16-
solid
liquid
0.
gas
100
200
temperature (K)
300
Note: your answer must be within 12.5 °C of the exact answer to be graded correct.
10
Explanation
Check
§
Q Search
J
2025 McGraw Hill LLC. All Rights Rese
151.2
254.8
85.9
199.6
241.4
87.6
242.5
186.4
155.8
257.1
242.9
253.3
256.0
216.6
108.7
239.0
149.7
236.4
152.1
222.7
148.7
278.2
268.7
234.4
262.7
283.2
143.6
QUESTION: Using this group of data on salt reduced tomato sauce concentration readings answer the following questions:
1. 95% Cl Confidence Interval (mmol/L)
2. [Na+] (mg/100 mL)
3. 95% Na+ Confidence Interval (mg/100 mL)
Chapter 31 Solutions
ORGANIC CHEMISTRY
Ch. 31 - Problem 31.1
One component of jojoba oil is a wax...Ch. 31 - Problem 31.2
How would you expect the melting...Ch. 31 - Problem 31.3
Draw the products formed when...Ch. 31 - Problem 31.4
The main fatty acid component of the...Ch. 31 - Prob. 31.5PCh. 31 - Problem 31.6
Draw the structure of a lecithin...Ch. 31 - Prob. 31.7PCh. 31 - Problem 31.8
Why are phospholipids, but not...Ch. 31 - Problem 31.9
Explain why regularly ingesting a...Ch. 31 - Problem 31.10
Locate the isoprene units in each...
Ch. 31 - Problem 31.11
Locate the isoprene units in...Ch. 31 - Problem 31.12
Write a stepwise mechanism for the...Ch. 31 - Prob. 31.13PCh. 31 - Prob. 31.14PCh. 31 - Prob. 31.15PCh. 31 - Prob. 31.16PCh. 31 - 31.17 Locate the isoprene units in each...Ch. 31 - Prob. 31.18PCh. 31 - Prob. 31.19PCh. 31 - Prob. 31.20PCh. 31 - Prob. 31.21PCh. 31 - 31.22 What is the structure of an optically...Ch. 31 - Prob. 31.23PCh. 31 - 31.24 Draw the structure of the following...Ch. 31 - Prob. 31.25PCh. 31 - Locate the isoprene units in each compound. a. e....Ch. 31 - 31.27 Classify each terpene and terpenoid in...Ch. 31 - 31.38 Draw the products formed when cholesterol is...Ch. 31 - 31.29 An isoprene unit can be thought of as having...Ch. 31 - 31.30 Draw a stepwise mechanism for the conversion...Ch. 31 - Prob. 31.31PCh. 31 - Prob. 31.32PCh. 31 - Draw three-dimensional structures f or each...Ch. 31 - Prob. 31.34PCh. 31 - Prob. 31.35PCh. 31 - Prob. 31.36PCh. 31 - Prob. 31.37PCh. 31 - 31.38 Draw the products formed when cholesterol is...Ch. 31 - 31.39 Draw a stepwise mechanism for the following...Ch. 31 - 31.40 Draw a stepwise mechanism for the following...Ch. 31 - Prob. 31.41P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Results Search Results Best Free Coursehero Unloc xb Success Confirmation of Q x O Google Pas alekscgi/x/lsl.exe/1o_u-IgNslkr 7j8P3jH-IQs_pBanHhvlTCeeBZbufu BYTI0Hz7m7D3ZcHYUt80XL-5alyVpwDXM TEZayFYCavJ17dZtpxbFD0Qggd1J O States of Matter Using a phase diagram to find a phase transition temperature or pressure Gabr 3/5 he pressure above a pure sample of solid Substance X at 101. °C is lowered. At what pressure will the sample sublime? Use the phase diagram of X below to nd your answer. pressure (atm) 24- 12 solid liquid gas 200 400 temperature (K) 600 ote: your answer must be within 0.15 atm of the exact answer to be graded correct. atm Thanation Check © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center I Q Search L³ ملةarrow_forward301.7 348.9 193.7 308.6 339.5 160.6 337.7 464.7 223.5 370.5 326.6 327.5 336.1 317.9 203.8 329.8 221.9 331.7 211.7 309.6 223.4 353.7 334.6 305.6 340.0 304.3 244.7 QUESTION: Using this group of data on regular tomato sauce concentration readings answer the following questions: 1. 95% Cl Confidence Interval (mmol/L) 2. [Na+] (mg/100 mL) 3. 95% Na+ Confidence Interval (mg/100 mL)arrow_forwardSearch Results Search Results Best Free Coursehero Unlo x b Success Confirmation of Q aleks.com/alekscgi/x/sl.exe/10_u-lgNslkr7j8P3jH-IQs_pBan HhvlTCeeBZbufu BYTIOHz7m7D3ZcHYUt80XL-5alyVpwDXM TEZayFYCav States of Matter Using a phase diagram to find a phase transition temperature or pressure Use the phase diagram of Substance X below to find the temperature at which X turns to a gas, if the pressure above the solid is 3.7 atm. pressure (atm) 0. 32- 16 solid liquid gas 200 temperature (K) Note: your answer must be within 20 °C of the exact answer to be graded correct. Дос Xarrow_forward
- Consider the reaction below to answer the following questions: Acetoacetic ester can be prepared by the Claisen self-condensation reaction of ethyl acetate. 1. NaOEt, EtOH H&C OCH CH3 2 H30 H3C CH2 OCH2CH3 A. Write the complete stepwise mechanism for this reaction. Show all electron flow with arrows and draw all intermediate structures. B. Ethyl acetate can be prepared from ethanol as the only organic starting material. Show all reagents and structures for all intermediates in this preparation. C. Give the structures of the ester precursors for the following Claisen condensation product and formulate the reaction. OEtarrow_forwardUse the phase diagram of Substance X below to find the temperature at which X turns to a gas, if the pressure above the solid is 3.7 atm. pressure (atm) 32 16 solid liquid gas 0 0 200 temperature (K) Note: your answer must be within 20 °C of the exact answer to be graded correct. Шос ☑ كarrow_forwardStarting from bromoethane, how could you prepare the following compounds: a. Ethanol. b. Acetaldehyde f. Acetone. e. 2-Propanol i. Acetoacetic ester. d. 2-Bromoacetic acid. c. Acetic acid g. Acetamide. j. Ethylmalonate k. Gama ketoacid. h. Ethyl magnesium bromide.arrow_forward
- - The pressure above a pure sample of solid Substance X at 60. °C is raised. At what pressure will the sample melt? Use the phase diagram of X below to find your answer. pressure (atm) 02 0.4 solid Hliquid gas 0 0 200 400 600 temperature (K) Note: your answer must be within 0.025 atm of the exact answer to be graded correct. ☐ atmarrow_forward15. What is the order of decreasing reactivity towards nucleophilic acyl substitution for the carboxylic acid derivatives? (most reactive first) 0 O H3C COC CH3 H₂C C N(CH3)2 H3C C OCH3 A. a. I, 11, 111, b. I, III, IV, II C. II, IV, III, I ° (CH3)2CH C OCH3 IV d. II, I, III, IV B. R COCR 0 0 0 13= RC NH2 RC OR RC CI === IV a. I, III, II, IV b. II, III, I, IV C. III, II, I, IV d. IV, I, III, IIarrow_forwardDraw the formula of the product obtained by reacting D-Tallose with bromine water.arrow_forward
- Choose the best reagent(s) for carrying out the following conversions from the list below. Place the letter corresponding to the best choice in the blank to the left of the conversion. a. KMnO4, H3O+ b. Tollens' Reagent [oxidizing reagent] C. NaBH4, ethanol d. 1. BH3 2. H3O+ e. 1. CH3MgBr, ether 2. H3O+ f. CrO3, H2SO4, H₂O g. 1. Mg, ether 2. CO2 3. H3O+ h. 1. NaCN 2. H2SO4, H2, heat i. O3, then Zn and HOAC j. CH₂I A. B. C. CH CH=CHCH2COOH Br CEN CH COOH + HOOCCH COOH COOH 010 CH3arrow_forwardDraw the structures for each of the intermediates in the boxes provided for the synthesis below. OCH3 Fe HO HNO (CHOO pynding H₂504 LHNO2 NACH-I Fa H₂O HCL HNO 180arrow_forwardProvide structure(s) for the starting material(s), reagent(s) or the major organic product(s) of each of the following reactions or sequences of reactions. Show all relevant stereochemistry [three only] A. o 11 (CH3)CH — C— C ether (CH3)2CH-C-O-C-CH3 B. CH3 CHy CI Staf OH C. HC OCHS + H₂Oarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:9781305081079
Author:STOKER, H. Stephen (howard Stephen)
Publisher:Cengage Learning,

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co
Lipids - Fatty Acids, Triglycerides, Phospholipids, Terpenes, Waxes, Eicosanoids; Author: The Organic Chemistry Tutor;https://www.youtube.com/watch?v=7dmoH5dAvpY;License: Standard YouTube License, CC-BY