
Organic Chemistry-Package(Custom)
4th Edition
ISBN: 9781259141089
Author: SMITH
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 30, Problem 30.31P
Draw a stepwise mechanism for the conversion of neryl diphosphate to
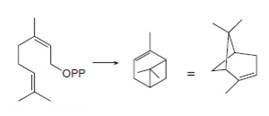
neryl diphosphate
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
PLEASE HELP! URGENT!
"Water gas" is an industrial fuel composed of a mixture of carbon monoxide and hydrogen gases. When this
fuel is burned, carbon dioxide and water result. From the information given below, write a balanced equation
and determine the enthalpy of this reaction:
CO(g) + O2(g) → CO₂(g) + 282.8 kJ
H2(g) + O2(g) → H₂O(g) + 241.8 kJ
MacBook Air
Page of 3
4. Calculate AG for the following reaction at 25°C. Will the reaction occur (be spontaneous)? How do you
know?
NH3(g) + HCl(g) → NH4Cl(s)
AH=-176.0 kJ
AS-284.8 J-K-1
Chapter 30 Solutions
Organic Chemistry-Package(Custom)
Ch. 30 - Problem 31.1
One component of jojoba oil is a wax...Ch. 30 - Prob. 30.2PCh. 30 - Problem 31.3
Draw the products formed when...Ch. 30 - Problem 31.4
The main fatty acid component of the...Ch. 30 - Prob. 30.5PCh. 30 - Problem 31.6
Draw the structure of a lecithin...Ch. 30 - Prob. 30.7PCh. 30 - Problem 31.8
Why are phospholipids, but not...Ch. 30 - Problem 31.9
Explain why regularly ingesting a...Ch. 30 - Prob. 30.10P
Ch. 30 - Problem 31.10
Locate the isoprene units in each...Ch. 30 - Prob. 30.12PCh. 30 - Problem 31.11
Locate the isoprene units in...Ch. 30 - Problem 31.12
Write a stepwise mechanism for the...Ch. 30 - Prob. 30.15PCh. 30 - Prob. 30.16PCh. 30 - Prob. 30.17PCh. 30 - Prob. 30.18PCh. 30 - 31.17 Locate the isoprene units in each...Ch. 30 - Prob. 30.20PCh. 30 - Prob. 30.21PCh. 30 - Prob. 30.22PCh. 30 - Prob. 30.23PCh. 30 - 31.22 What is the structure of an optically...Ch. 30 - Prob. 30.25PCh. 30 - 31.24 Draw the structure of the following...Ch. 30 - Prob. 30.27PCh. 30 - Locate the isoprene units in each compound. a. e....Ch. 30 - Classify each terpene and terpenoid in Problem...Ch. 30 - 31.29 An isoprene unit can be thought of as having...Ch. 30 - 31.30 Draw a stepwise mechanism for the conversion...Ch. 30 - Prob. 30.32PCh. 30 - Prob. 30.33PCh. 30 - Draw three-dimensional structures f or each...Ch. 30 - Prob. 30.35PCh. 30 - Prob. 30.36PCh. 30 - Prob. 30.37PCh. 30 - 31.38 Draw the products formed when cholesterol is...Ch. 30 - 31.39 Draw a stepwise mechanism for the following...Ch. 30 - 31.40 Draw a stepwise mechanism for the following...Ch. 30 - Prob. 30.41P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- true or false The equilibrium constant for this reaction is 0.20. N2O4(g) ⇔ 2NO2(g) Based on the above, the equilibrium constant for the following reaction is 5. 4NO2(g) ⇔ 2N2O4(g)arrow_forwardtrue or false The equilibrium constant for this reaction is 0.20. N2O4(g) ⇔ 2NO2(g) Based on the above, the equilibrium constant for the following reaction is 0.4. 2N2O4(g) ⇔ 4NO2(g)arrow_forwardtrue or false Using the following equilibrium, if heat is added the equilibrium will shift toward the reactants. N2(g) + 3H2(g) ⇔ 2NH3(g) + heatarrow_forward
- True or False Using the following equilibrium, if heat is added the equilibrium will shift toward the products. N2O4(g) + heat ⇔ 2NO2(g)arrow_forwardtrue or false Using the following equilibrium, if solid carbon is added the equilibrium will shift toward the products. C(s) + CO2(g) ⇔ 2CO(g)arrow_forwardProvide the complete mechanism for the reaction below. You must include appropriate arrows,intermediates, and formal charges. Please also provide a reason to explain why the 1,4-adduct is preferred over the 1,3-adduct.arrow_forward
- Which of the following pairs are resonance structures of one another? I. III. || III IV + II. :0: n P !༠ IV. EN: Narrow_forwardPredict the major organic product(s) and byproducts (either organic or inorganic) for thefollowing reactions.arrow_forwardA 8.25 g sample of aluminum at 55°C released 2500 J of heat. The specific heat of aluminum is 0.900 J/g°C. The density of aluminum is 2.70 g/mL. Calculate the final temperature of the aluminum sample in °C.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
Lipids - Fatty Acids, Triglycerides, Phospholipids, Terpenes, Waxes, Eicosanoids; Author: The Organic Chemistry Tutor;https://www.youtube.com/watch?v=7dmoH5dAvpY;License: Standard YouTube License, CC-BY