![OWLv2 for Moore/Stanitski's Chemistry: The Molecular Science, 5th Edition, [Instant Access], 1 term (6 months)](https://s3.amazonaws.com/compass-isbn-assets/textbook_empty_images/large_textbook_empty.svg)
Concept explainers
In a blast furnace at high temperature, iron(III) oxide in ore reacts with carbon monoxide to produce metallic iron and carbon dioxide. The liquid iron produced is cooled and weighed. The reaction is run repeatedly with the same initial mass of iron(III) oxide, 19.0 g, but differing initial masses of carbon monoxide. The masses of iron obtained arc shown in this graph.
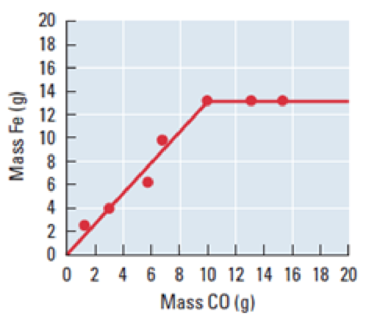
- (a) Write the balanced chemical equation for this reaction.
- (b) Calculate the mass of CO required to react completely with 19.0 g iron(III) oxide.
- (c) Calculate the mass of carbon dioxide produced when the reaction converts 10.0 g iron(III) oxide completely to products.
- (d) From the graph, determine which reactant is limiting when less than 10.0 g carbon monoxide is available to react with 19.0 g iron(III) oxide.
- (e) From the graph, determine which reactant is limiting when more than 10.0 g carbon monoxide is available to react with 19.0 g iron(III) oxide.
- (f) Calculate the percent yield if 24.0 g iron(III) oxide reacted with 20.0 g carbon monoxide to produce 15.9 g metallic iron.
- (g) Calculate the minimum mass of additional limiting reactant required to react with all of the excess of nonlimiting reactant from part (f).
(a)
Interpretation:
Iron
For the given reaction, balanced equation has to be written.
Concept introduction:
Balanced Chemical equation:
A balanced chemical equation is an equation which contains same elements in same number on both the sides (reactant and product side) of the chemical equation thereby obeying the law of conservation of mass.
Balancing the equation:
- There is a Law for conversion of mass in a chemical reaction i.e., the mass of total amount of the product should be equal to the total mass of the reactants.
- First write the skeletal reaction from the given information.
- Then count the number of atoms of each element in reactants as well as products.
- Place suitable coefficients in front of reactants as well as products until the number of atoms on each side (reactants and products) becomes equal.
Explanation of Solution
Given,
While balancing the equation, the subscripts cannot be altered but coefficients can be changed. There are two iron atoms in the left side and one iron atom in the right side of the reaction. Therefore, two iron atoms are added to right side of reaction. Now, the balanced equation is given below.
Three molecule of carbon monoxide is added to the left side of the reaction and three molecules of carbon dioxide is added to the right side of the reaction. Now, the balanced equation is given below.
(b)
Interpretation:
Mass of
Explanation of Solution
Given,
The molar mass of
According to the balancing equation, one mole of
Therefore, mass of
Mass of
(c)
Interpretation:
Mass of carbon dioxide produced when the reaction converts
Explanation of Solution
Given,
The molar mass of
According to the balancing equation, one mole of
Therefore, mass of
Mass of
(d)
Interpretation:
When less than
Concept introduction:
A limiting reactant is a reactant that is completely converted to products. Once all the limiting reactant is converted to products there is no other reactant to react.
Explanation of Solution
Given,
![OWLv2 for Moore/Stanitski's Chemistry: The Molecular Science, 5th Edition, [Instant Access], 1 term (6 months), Chapter 3, Problem IISP , additional homework tip 1](https://content.bartleby.com/tbms-images/9781285199047/Chapter-3/images/99047-3-iisp_image_001.png)
Figure 1
One mole of
The given graph between the mass of Fe and mass of carbon monoxide. The graph tells that the weight of the carbon monoxide is below
The calculation, shows that
(e)
Interpretation:
When more than
Concept introduction:
Refer to part (d)
Explanation of Solution
Given,
![OWLv2 for Moore/Stanitski's Chemistry: The Molecular Science, 5th Edition, [Instant Access], 1 term (6 months), Chapter 3, Problem IISP , additional homework tip 2](https://content.bartleby.com/tbms-images/9781285199047/Chapter-3/images/99047-3-iisp_image_002.png)
Figure 1
One mole of
The given graph between the mass of Fe and mass of carbon monoxide. The graph tells that the weight of the carbon monoxide is below
The graph tells that the weight of the carbon monoxide is more than
The calculation,
(f)
Interpretation:
When 24.0 g iron
Concept introduction:
The percent yield can be calculated by using following formula
Explanation of Solution
Given,
The molar mass of
According to the balancing equation, one mole of
Given,
Number of moles of iron is calculated as follows,
Number of moles of iron is calculated as follows,
According to the mole calculation,
Amount of
The percent yield can be calculated by using following formula
The percent yield is
(g)
Interpretation:
The minimum mass of additional limiting reactant necessary to react with all of the excess of non-limiting reactant has to be calculated.
Explanation of Solution
Given,
The molar mass of
According to the balancing equation, one mole of
Given,
Number of moles of iron is calculated as follows,
Number of moles of iron is calculated as follows,
According to the mole calculation,
Gram of
Want to see more full solutions like this?
Chapter 3 Solutions
OWLv2 for Moore/Stanitski's Chemistry: The Molecular Science, 5th Edition, [Instant Access], 1 term (6 months)
- If the symbol A is placed in a reaction, at what temperature does it take place?arrow_forwardBy malonic or acetylacetic synthesis, synthesize 3-methyl-4-oxopentanoic acid (indicate the formulas of the compounds).arrow_forwardoalmitic acid is a 16 carbon acid. In a balanced equation, the products of the sponification of tripalmitin (glyceryl tripalmitate are blank.arrow_forward
- Write the esterification reaction mechanism of salicylic acid and acetic acid to produce aspirin (acetylsalicylic acid). Note: salicylic acid will act as the alcoholarrow_forwardWhat type of interaction would you expect between the following R groups in the tertiary structure of a protein? O -CH2-CO and -CH2-CH2-CH2-CH2-NH3+ a. disulfide bonds b. salt bridges c. hydrogen bonds HO abios vist anisinoo tedt bigil s ai loistaslor sale! 10 OUT d. hydrophobic interactions e. peptide bondsarrow_forward4. True or false: This skeletal structure represents a saturated fatty acid. Ini to 0 fale) me OH faistong starrow_forward
- By malonic or acetylacetic synthesis, synthesize 5-Methyl-2-hexanone (with the formulas of the compounds).arrow_forwardQUESTION: Answer Question 5: 'Calculating standard error of regression' by filling in all the empty green boxes *The values are all provided in the first photo attached*arrow_forwardDraw the formula for 3-chlorobenzoic acetic anhydride.arrow_forward
- By malonic or acetylacetic synthesis, synthesize 2-methylbutanoic acid (indicate the formulas of the compounds).arrow_forwardObtain 2-methylbutanoic acid by malonic or acetylacetic synthesis (indicate the formulas of the compounds involved).arrow_forwardEFFICIENTS SAMPLE READINGS CONCENTRATIONS Pigiadient) TOMATO SAUCE (REGULAR) TOMATO (REDUCED SALT) TOMATO SAUCE (REGULAR) TOMATO (REDUCED SALT) 58 6.274 3.898 301.7 151.2 14150 5.277 3.865 348.9 254.8 B 5.136 3.639 193.7 85.9 605 4.655 3.041 308.6 199.6 05 5.135 3.664 339.5 241.4 0139 4.676 3.662 160.6 87.6 90148 5.086 3.677 337.7 242.5 0092 6.348 3.775 464.7 186.4 PART3 5.081 3.908 223.5 155.8 5.558 3.861 370.5 257.1 4.922 3.66 326.6 242.9 4.752 3.641 327.5 253.3 50 5.018 3.815 336.1 256.0 84 4.959 3.605 317.9 216.6 38 4.96 3.652 203.8 108.7 $3 5.052 3.664 329.8 239.0 17 5.043 3.767 221.9 149.7 052 5.058 3.614 331.7 236.4 5.051 4.005 211.7 152.1 62 5.047 3.637 309.6 222.7 5.298 3.977 223.4 148.7 5.38 4.24 353.7 278.2 5 5.033 4.044 334.6 268.7 995 4.706 3.621 305.6 234.4 04 4.816 3.728 340.0 262.7 16 4.828 4.496 304.3 283.2 0.011 4.993 3.865 244.7 143.6 AVERAGE STDEV COUNT 95% CI Confidence Interval (mmol/L) [Na+] (mg/100 mL) 95% Na+ Confidence Interval (mg/100 mL)arrow_forward
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning





