
Concept explainers
MATHEMATICAL Predict the predominant forms of the amino acids from Question 8 at pH 10.
Interpretation:
The predominant forms of amino acids, histidine, asparagine, tryptophan, proline, and tyrosine, at pH 10 are to be predicted.
Concept introduction:
The name ‘amino acids’ itself implies that they have the amino group and carboxylic acid group. The central atom of the amino acid is called the alpha-carbon and that is why all the amino acids are called alpha-amino acids.
The naturally occurring amino acids are alpha-amino acids. The amino group attains the positive charge and the carboxyl group attains the negative charge at the neutral pH (potential of hydrogen).
Answer to Problem 9RE
Solution:
The imidazole ring of the histidine amino acid is deprotonated and the
The
The
The
The
Explanation of Solution
The imidazole ring in the side chain of the histidine amino acid remains partially protonated in the physiological condition, which classifies the amino acid as the positively charged amino acid and keeps this amino acid in the polar amino acid category.
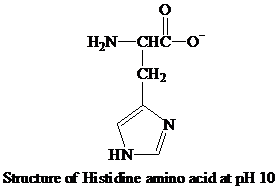
The histidine loses the proton ions from the imidazole ring and the
The

When the pH increases and reaches to 10, the
The
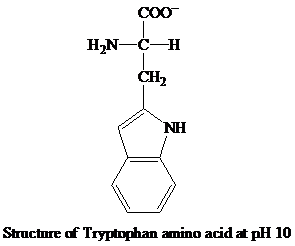
Then, the amino group becomes neutral and the carboxyl group attains the negative charge. The negative charge of the carboxyl group contributes to the anionic behavior of the tryptophan amino acid at the pH 10.
The proline remains positively-charged at the acidic pH as the amino group is having the proton ion, and as the pH increases, the deprotonation occurs and the carboxyl group loses the proton ion and becomes negatively-charged.

The positive charge and the negative charge cancel out the effect of each other and thus, the whole amino acid becomes neutral in nature at the pH 10.
The tyrosine has the phenyl group in the side chain and hydroxyl group is also present at the phenyl group. The phenolic hydroxyl is 50% mixture of the protonated and deprotonated forms.

The
Therefore, it can be concluded that the histidine becomes anionic, asparagine becomes anionic, tryptophan becomes anionic, proline becomes neutral, and tyrosine becomes anionic at the pH 10.
(a)
Anion
(b)
Anion
(c)
Anion
(d)
Neutral
(e)
Anion
Explanation:
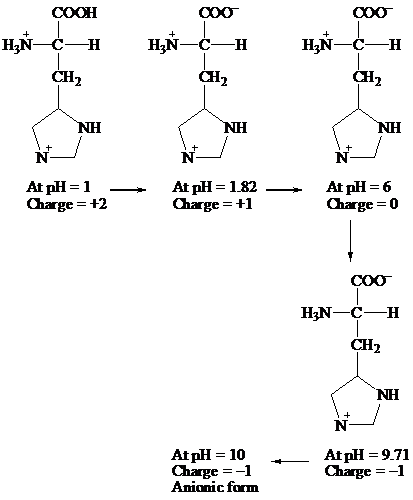
COOH COO- COO-
׀ ׀ ׀
H3N+ — C — H H3N+ — C — H H3N+ — C — H
׀ ׀ ׀
CH2 CH2 CH2
׀ ׀ ׀

At pH = 1 → At pH= 1.82 → At pH = 6
Charge = +2 Charge = +1 Charge = 0
↓
COO-
H3N— C — H
׀
CH2
׀

At pH = 10 ← At pH = 9.71
Charge = -1 Charge = -1
Anionic form
Hence, the predominant form of histidine at pH 10 is anionic.
___________________________________________________________________________
(b)
Given information: Asparagine at pH 10
Explanation:
COOH COO- COO-
׀ ׀ ׀
H3N+ — C — H H3N+ — C — H H2N — C — H
׀ | |
CH2 CH2 CH2
׀ ׀ ׀
C = O C = O C = O
׀ ׀ ׀
NH2 NH2 NH2
At pH = 1 → At pH= 2.02 → At pH = 8.80 → At pH = 10
Charge = +1 Charge = 0 Charge = -1 Charge = -1
Anionic
form
Hence, the predominant form of asparagine at pH 10 is anionic.
___________________________________________________________________________
(c)
Given information: Tryptophan at pH 10
Explanation:
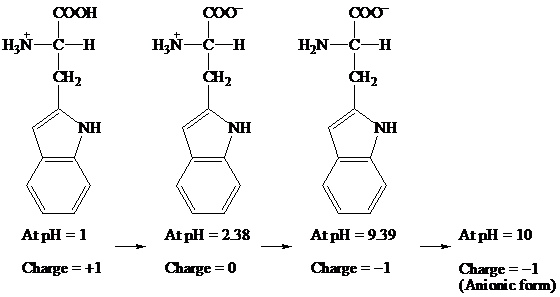
COOH COO- COO-
׀ ׀ ׀
H3N+ — C — H H3N+ — C — H H2N — C — H
׀ ׀ ׀
CH2 CH2 CH2
׀ ׀ ׀


At pH = 1 → At pH= 2.38 → At pH = 9.39 → At pH = 10
Charge = +1 Charge = 0 Charge = -1 Charge = -1
(Anionic form)
Hence, the predominant form of tryptophan at pH 10 is anionic.
___________________________________________________________________________
(d)
Given information: Proline at pH 10.
Explanation:
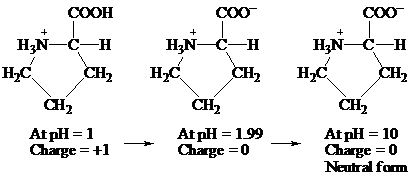
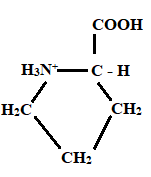
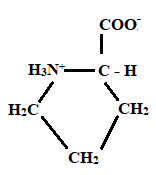

At pH = 1 → At pH= 1.99 → At pH = 10
Charge = +1 Charge = 0 Charge = 0
Neutral form
Hence, the predominant form of proline at pH 10 is neutral.
___________________________________________________________________________
(e)
Given information: Tyrosine at pH 10.
Explanation:
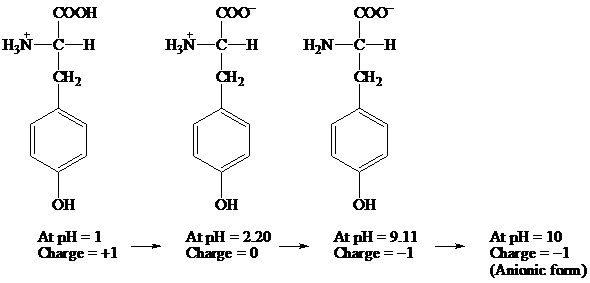
COOH COO- COO-
׀ ׀ ׀
H3N+ — C — H H3N+ — C — H H2N — C — H
׀ ׀ ׀
CH2 CH2 CH2
׀ ׀ ׀


At pH = 1 → At pH= 2.20 → At pH = 9.11 → At pH = 10
Charge = +1 Charge = 0 Charge = -1 Charge = -1
(Anionic form)
Hence, the predominant form of tyrosine at pH 10 is anionic.
Want to see more full solutions like this?
Chapter 3 Solutions
BIOCHEMISTRY (LOOSELEAF) >CUSTOM PKG<
- Show the fate of the proton on the 4-Oxygen molecule of F-1,6-BP.arrow_forwardSodium borohydride (NaBH4) is a potent inhibitor of aldolase. It is known to ONLY inhibit theenzyme when it is complexed with substrate. Treatment of the enzyme alone has no effect.What is the mechanism for this inhibition?arrow_forwardA non-hydrolysable ATP (AMPPNP - below) is ingested by a graduate student on a dare. Whateffects would you anticipate on his metabolism?arrow_forward
- Show the mechanism for the acid-catalyzed formation of an [α-1,6] glycosidic linkagebetween two molecules of α-D-glucopyranose. Please sketch the structure and use arrows showing electron flow!arrow_forwardI am a Biochemistry student and I am confused on how to analyze FRAP Analysis using Excel Spread Sheets. The following spread sheet has my 0 minute data listed at top and the 4 minute data listed on the bottom. Sheet: https://mnscu-my.sharepoint.com/:x:/g/personal/vi2163ss_go_minnstate_edu/EXjrCizWiXRPmpittqZA12IB8EkB5eE8iaRqj_iun-IAtg?rtime=Wo9zPHFY3Ug The formula for FRAP Analysis is: FRAP value = A (4 min sample) - A (0 min sample) over A (4 min 30 uM ascorbic acid) - A (0 min 30 uM ascorbic acid) multiplied by 30 uM and the dilution factor of 1/10arrow_forwardHO Fill in the missing boxes. ON 800 NO NO Glucose ATP NADH Hexokinase 1,3-Bisphosphoglycerate Mg2+ ADP NAD+, Pi Phosphoglucose Isomerase Glucose-6-Phosphate ON 沁 Glyceraldehyde-3-Phosphate HO حلمة ADP ADP Phospho Mg2+ glycerate Dihydroxyacetone Phosphate ATP kinase ATP Phosphoglycerate 3-phosphoglycerate Mutase H₁₂O Fructose-6-Phosphate ATP Mg2+ ADP Fructose-1,6-Bisphosphate 2-phosphoglycerate H₂O Phosphoenolpyruvate ADP Mg2+ ATP Pyruvatearrow_forward
- In a diffraction experiment of a native crystal, intensity of reflection (-1 0 6) is equivalent to the intensity of reflection (1 0 -6). true or false?arrow_forwardin an x-ray diffraction experiment, moving the detector farther away from the crystal will allow collection of reflection of reflections with high Miller indices. true or false?arrow_forwardShow the mechanism for the acid-catalyzed formation of an [α-1,6] glycosidic linkagebetween two molecules of α-D-glucopyranose.arrow_forward
- Label the following polysaccharide derivatives as reducing or nonreducing. a. C. b. HO CH₂OH CH2OH OH OH OH OH OH HOCH₂ OH OH OH HOCH₂ HO HO HO OH OH ΙΟ CH₂OH OH OH "OH OHarrow_forwardFor a red blood cell (erythrocyte) undergoing active glycolysis, briefly explain how increases in concentration of the following factors are likely to affect glycolytic flux. a. ATP b. AMP c. F-1,6-BP d. F-2,6-BP e. Citrate f. Glucose-6-phosphatearrow_forwardThe ∆G°’ for hydrolysis of phosphoenol pyruvate is -62.2 kJ/mol. The standard freeenergy of ATP hydrolysis is -30.5 kJ/mol. A. What is the standard free energy and K eq of the spontaneous reaction betweenADP/ATP and phosphoenol pyruvate. B. Repeat A for F-1,6-BP (∆G°’=-16.7 kJ/mol) and 1,3-BPG (∆G°’=-49.6 kJ/mol)hydrolysis. C. If the ATP and ADP concentrations are 8mM and 1mM respectively, what would bethe ratio of pyruvate/phosphoenolpyruvate at equilibrium?arrow_forward
 BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
