
Custom eBook for Organic Chemistry
2nd Edition
ISBN: 9798214171104
Author: Straumanis
Publisher: Cengage Custom
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 3, Problem 4E
Consider the incomplete orbital representation of
a. Identify which lobes are hybrid orbitals (identify the type) and which lobes arep orbitals.
b. Use dotted lines to show any
c. Use up or down arrows to show electron occupation of each hybrid orbital or bond.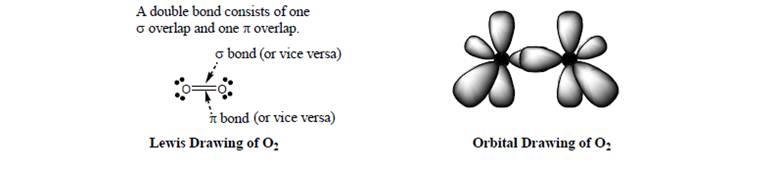
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
3. Enter the molecular weight of the product obtained from the Williamson Ether Synthesis?
OH OH
&
OH
excess CH3l
Ag₂O
Please answer 1, 2 and 3 on the end
In the box below, specify which of the given compounds are very soluble in polar aprotic solvents. You may select more than one compound. Choose one or more: NaCl NH4Cl CH3CH2CH2CH2CH2CN CH3CH2OH hexan-2-one NaOH CH3SCH3
Chapter 3 Solutions
Custom eBook for Organic Chemistry
Ch. 3 - Prob. 1CTQCh. 3 - What neutral atom is represented by the electron...Ch. 3 - Prob. 3CTQCh. 3 - Consider any one of the four identical hybrid...Ch. 3 - Prob. 5CTQCh. 3 - Prob. 6CTQCh. 3 - Prob. 7CTQCh. 3 - Prob. 8CTQCh. 3 - Prob. 9CTQCh. 3 - Prob. 10CTQ
Ch. 3 - On the left side of Figure 3.6, label the areas...Ch. 3 - Prob. 12CTQCh. 3 - Prob. 13CTQCh. 3 - Prob. 14CTQCh. 3 - Prob. 15CTQCh. 3 - Now consider the fully formed molecule on the...Ch. 3 - Prob. 1ECh. 3 - Explain why the two molecules below cannot...Ch. 3 - Prob. 3ECh. 3 - Consider the incomplete orbital representation of...Ch. 3 - Consider the following orbital representation of...Ch. 3 - Summarize how one determines the hybridization...Ch. 3 - Explain what is wrong with each of the following...Ch. 3 - Prob. 8ECh. 3 - Prob. 9ECh. 3 - Complete the following tables, and memorize their...Ch. 3 - Draw orbital representations of bonding in water...Ch. 3 - Draw electron configuration diagrams for carbon in...Ch. 3 - Prob. 13E
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- On the following structure, select all of the atoms that could ACCEPT a hydrogen bond. Ignore possible complications of aromaticity. When selecting be sure to click on the center of the atom.arrow_forwardRank the compounds below from lowest to highest melting point.arrow_forward18 Question (1 point) Draw the line structure form of the given partially condensed structure in the box provided. :ÖH HC HC H2 ΙΩ Н2 CH2 CH3 CH3 partially condensed formarrow_forward
- someone else has already submitted the same question on here and it was the incorrect answer.arrow_forwardThe reaction: 2NO2(g) ⇌ N2O4(g) is an exothermic reaction, ΔH=-58.0 kJ/molrxn at 0°C the KP is 58.If the initial partial pressures of both NO2(g) and N2O4(g) are 2.00 atm:A) Is the reaction at equilibrium? If not, what is the value of Q? B) Which direction will the reaction go to reach equilibrium? C) Use an ICE table to find the equilibrium pressures.arrow_forwardThe dissociation of the weak acid, nitrous acid, HNO2, takes place according to the reaction: HNO2 (aq) ⇌ H+(aq) + NO2–(aq) K=7.2 X 10-4 When 1.00 mole of HNO2 is added to 1.00 L of water, the H+ concentration at equilibrium is 0.0265 M.A) Calculate the value of Q if 1.00 L of water is added? B) How will reaction shift if 1.00 L of water is added?arrow_forward
- Suppose a certain copolymer elastomeric material “styrene-butadiene rubber”) contains styrene ("S") monomers –(C8H8)– and butadiene ("B") monomers –(C4H6)– and that their numerical ratio S:B = 1:8. What is the mass ratio mS:mB of the two monomers in the material? What is the molecular mass M of a macromolecule of this copolymer with degree of polymerization n = 60,000? Data: AC = 12.01 u, AH = 1.008 u.arrow_forwardLab Questions from Lab: Gravimetric Determination of Calcium as CaC2O4•H2O What is the purpose of the methyl red indicator? Why does a color change to yellow tell you that the reaction is complete? Why is the precipitate rinsed with ice-cold water in step 4? Why not room temperature or hot water? Why is it important that the funnels be placed in a desiccator before weighing (steps 1 and 5)?arrow_forwardWhat mass of ethylene glycol, HOCH2CH2OH, Mustbe added to 5.50 kg of water to antifreeze that would work for the car radiator to -10.0 degrees celcius? MM (g/mol): 62.07arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
Stoichiometry - Chemistry for Massive Creatures: Crash Course Chemistry #6; Author: Crash Course;https://www.youtube.com/watch?v=UL1jmJaUkaQ;License: Standard YouTube License, CC-BY
Bonding (Ionic, Covalent & Metallic) - GCSE Chemistry; Author: Science Shorts;https://www.youtube.com/watch?v=p9MA6Od-zBA;License: Standard YouTube License, CC-BY
General Chemistry 1A. Lecture 12. Two Theories of Bonding.; Author: UCI Open;https://www.youtube.com/watch?v=dLTlL9Z1bh0;License: CC-BY