
Essential Organic Chemistry, Global Edition
3rd Edition
ISBN: 9781292089034
Author: Paula Yurkanis Bruice
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 3, Problem 45P
What is each compound’s name?
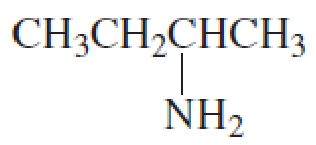
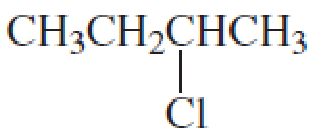
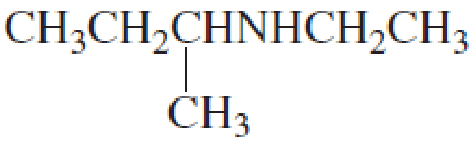
- a. CH3CH2CH2OCH2CH3
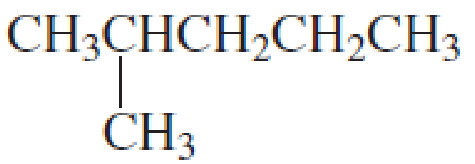
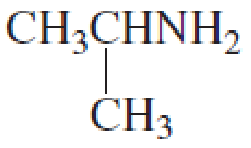
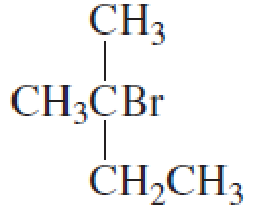
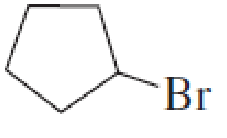
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Indicate the product(s) A, B C and D that are formed in
the reaction:
H
+ NH-NH-CH
[A+B]
[C+D]
hydrazones
How can you prepare a 6 mL solution of 6% H2O2, if we have a bottle of 30% H2O2?
How many mL of H2O2 from the 30% bottle must be collected to prepare 6 mL of 6% H2O2.
Chapter 3 Solutions
Essential Organic Chemistry, Global Edition
Ch. 3.1 - Name each of the following:Ch. 3.1 - Draw the structures and name the four...Ch. 3.1 - Draw the structure for each of the following...Ch. 3.1 - Name the following compounds:Ch. 3.1 - Prob. 7PCh. 3.2 - What is each compounds systematic name?Ch. 3.2 - Prob. 11PCh. 3.3 - Prob. 12PCh. 3.3 - Convert the following condensed structures into...Ch. 3.3 - The molecular formula for ethyl alcohol (CH3CH2OH)...
Ch. 3.3 - Draw a condensed and a skeletal structure for the...Ch. 3.3 - What is each compounds systematic name?Ch. 3.4 - Prob. 17PCh. 3.4 - Give two names for each of the following alkyl...Ch. 3.5 - Are the following compounds primary, secondary, or...Ch. 3.5 - Name the following amines and tell whether they...Ch. 3.5 - Draw the structures and provide systematic names...Ch. 3.6 - Predict the approximate size of the following bond...Ch. 3.7 - What is the smallest straight-chain alkane that is...Ch. 3.7 - Prob. 24PCh. 3.7 - Prob. 25PCh. 3.7 - Prob. 26PCh. 3.7 - List the compounds in each set from highest...Ch. 3.8 - Rank the following compounds in each set from most...Ch. 3.8 - In which solvent would cyclohexane have the lowest...Ch. 3.8 - Prob. 30PCh. 3.9 - Prob. 31PCh. 3.9 - a. Draw the three staggered conformations and the...Ch. 3.9 - a. Draw the most stable conformation of pentane...Ch. 3.11 - Draw 1,2,3,4,5,6-hexachlorocyclohexane with a. all...Ch. 3.12 - At any one time, would you expect there to be more...Ch. 3.13 - Prob. 36PCh. 3 - a. How many hydrogens does an alkane with 17...Ch. 3 - Prob. 2PCh. 3 - Draw a condensed structure and a skeletal...Ch. 3 - Prob. 39PCh. 3 - Prob. 40PCh. 3 - Which of the following represents a cis isomer?Ch. 3 - a. How many primary carbons does each of the...Ch. 3 - Name the following amines and tell whether they...Ch. 3 - Which of the following conformers of isobutyl...Ch. 3 - What is each compounds name? a. CH3CH2CH2OCH2CH3Ch. 3 - Draw the structural formula for an alkane that has...Ch. 3 - Which has a. the higher boiling point:...Ch. 3 - Ansaid and Motrin belong to the group of drugs...Ch. 3 - A student was given the structural formulas of...Ch. 3 - Which of the following conformers has the highest...Ch. 3 - Prob. 51PCh. 3 - Draw skeletal structures for the following: a....Ch. 3 - Prob. 53PCh. 3 - For rotation about the C-3 8 C-4 bond of...Ch. 3 - Prob. 55PCh. 3 - What is each compounds systematic name?Ch. 3 - Draw the two chair conformers for each of the...Ch. 3 - Draw the nine constitutional isomers with...Ch. 3 - Prob. 59PCh. 3 - Prob. 60PCh. 3 - Prob. 61PCh. 3 - Using Newman projections, draw the most stable...Ch. 3 - For each of the following disubstituted...Ch. 3 - Prob. 64PCh. 3 - Prob. 65PCh. 3 - What is each compounds systematic name?Ch. 3 - Prob. 67PCh. 3 - Bromine is a larger atom than chlorine, but the...Ch. 3 - Prob. 69PCh. 3 - Prob. 70PCh. 3 - a. Draw a potential energy diagram for rotation...
Additional Science Textbook Solutions
Find more solutions based on key concepts
2. Which of the following is the best example of the use of a referent? _
a. A red bicycle
b. Big as a dump tru...
Physical Science
To test your knowledge, discuss the following topics with a study partner or in writing ideally from memory. Th...
HUMAN ANATOMY
11. In the early 1800s, French naturalist Jean Baptiste Lamarck suggested that the best explanation for the rel...
Campbell Biology: Concepts & Connections (9th Edition)
More than one choice may apply. Using the terms listed below, fill in the blank with the proper term. anterior ...
Essentials of Human Anatomy & Physiology (12th Edition)
What process causes the Mediterranean intermediate Water MIW to become more dense than water in the adjacent At...
Applications and Investigations in Earth Science (9th Edition)
An obese 55-year-old woman consults her physician about minor chest pains during exercise. Explain the physicia...
Biology: Life on Earth with Physiology (11th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Indicate the product(s) B and C that are formed in the reaction: HN' OCH HC1 B + mayoritario C minoritario OCH3arrow_forwardIndicate the product(s) that are formed in the reaction: NH-NH, OCH3 -H₂O OCH3arrow_forward21.38 Arrange the molecules in each set in order of increasing acidity (from least acidic to most acidic). OH OH SH NH2 8 NH3 OH (b) OH OH OH (c) & & & CH3 NO2 21.39 Explain the trends in the acidity of phenol and the monofluoro derivatives of phenol. OH OH OH OH PK 10.0 PK 8.81 PK 9.28 PK 9.81arrow_forward
- identify which spectrum is for acetaminophen and which is for phenacetinarrow_forwardThe Concept of Aromaticity 21.15 State the number of 2p orbital electrons in each molecule or ion. (a) (b) (e) (f) (c) (d) (h) (i) DA (k) 21.16 Which of the molecules and ions given in Problem 21.15 are aromatic according to the Hückel criteria? Which, if planar, would be antiaromatic? 21.17 Which of the following structures are considered aromatic according to the Hückel criteria? ---0-0 (a) (b) (c) (d) (e) (h) H -H .8.0- 21.18 Which of the molecules and ions from Problem 21.17 have electrons donated by a heteroatom?arrow_forward1. Show the steps necessary to make 2-methyl-4-nonene using a Wittig reaction. Start with triphenylphosphine and an alkyl halide. After that you may use any other organic or inorganic reagents. 2. Write in the product of this reaction: CH3 CH₂ (C6H5)₂CuLi H₂O+arrow_forward
- 3. Name this compound properly, including stereochemistry. H₂C H3C CH3 OH 4. Show the step(s) necessary to transform the compound on the left into the acid on the right. Bri CH2 5. Write in the product of this LiAlH4 Br H₂C OHarrow_forwardWhat are the major products of the following reaction? Please provide a detailed explanation and a drawing to show how the reaction proceeds.arrow_forwardWhat are the major products of the following enolate alkylation reaction? Please include a detailed explanation as well as a drawing as to how the reaction proceeds.arrow_forward
- A block of zinc has an initial temperature of 94.2 degrees celcius and is immererd in 105 g of water at 21.90 degrees celcius. At thermal equilibrium, the final temperature is 25.20 degrees celcius. What is the mass of the zinc block? Cs(Zn) = 0.390 J/gxdegrees celcius Cs(H2O) = 4.18 J/gx degrees celcusarrow_forwardPotential Energy (kJ) 1. Consider these three reactions as the elementary steps in the mechanism for a chemical reaction. AH = -950 kJ AH = 575 kJ (i) Cl₂ (g) + Pt (s) 2C1 (g) + Pt (s) Ea = 1550 kJ (ii) Cl (g)+ CO (g) + Pt (s) → CICO (g) + Pt (s) (iii) Cl (g) + CICO (g) → Cl₂CO (g) Ea = 2240 kJ Ea = 2350 kJ AH = -825 kJ 2600 2400 2200 2000 1800 1600 1400 1200 1000 a. Draw the potential energy diagram for the reaction. Label the data points for clarity. The potential energy of the reactants is 600 kJ 800 600 400 200 0 -200- -400 -600- -800- Reaction Progressarrow_forwardCan u help me figure out the reaction mechanisms for these, idk where to even startarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning

Lipids - Fatty Acids, Triglycerides, Phospholipids, Terpenes, Waxes, Eicosanoids; Author: The Organic Chemistry Tutor;https://www.youtube.com/watch?v=7dmoH5dAvpY;License: Standard YouTube License, CC-BY