
Essential Organic Chemistry, Global Edition
3rd Edition
ISBN: 9781292089034
Author: Paula Yurkanis Bruice
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 3, Problem 42P
- a. How many primary carbons does each of the following compounds have?
- b. How many secondary carbons does each one have?
- c. How many tertiary carbons does each one have?
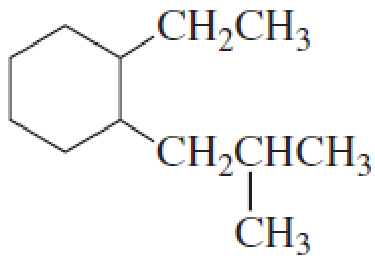
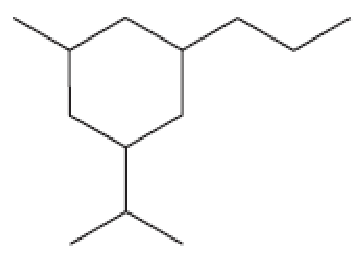
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
a. Draw 2 structures (kekule or skeletal) for C6H₁00₂ that has only sp³ carbons
b. Draw 2 structures (kekule or skeletal) for C6H₁00₂ that has only sp³ and sp² carbons
c. Draw 2 structures (kekule or skeletal) for C6H₁00₂ that has only sp³ and sp carbons
Determine if each compound is a. constitutional isomers b. enantionmers c. diastereomers d. the same molecule
1. Name each compound and determine the relationship between the pairs of compounds.
O
a. Name:
b. Name:
N
C. Relationship between structures above: Conformational isomer or Structural isomer
2. Name:
3. Is the following structure the cis stereoisomer or the trans stereoisomer?
Chapter 3 Solutions
Essential Organic Chemistry, Global Edition
Ch. 3.1 - Name each of the following:Ch. 3.1 - Draw the structures and name the four...Ch. 3.1 - Draw the structure for each of the following...Ch. 3.1 - Name the following compounds:Ch. 3.1 - Prob. 7PCh. 3.2 - What is each compounds systematic name?Ch. 3.2 - Prob. 11PCh. 3.3 - Prob. 12PCh. 3.3 - Convert the following condensed structures into...Ch. 3.3 - The molecular formula for ethyl alcohol (CH3CH2OH)...
Ch. 3.3 - Draw a condensed and a skeletal structure for the...Ch. 3.3 - What is each compounds systematic name?Ch. 3.4 - Prob. 17PCh. 3.4 - Give two names for each of the following alkyl...Ch. 3.5 - Are the following compounds primary, secondary, or...Ch. 3.5 - Name the following amines and tell whether they...Ch. 3.5 - Draw the structures and provide systematic names...Ch. 3.6 - Predict the approximate size of the following bond...Ch. 3.7 - What is the smallest straight-chain alkane that is...Ch. 3.7 - Prob. 24PCh. 3.7 - Prob. 25PCh. 3.7 - Prob. 26PCh. 3.7 - List the compounds in each set from highest...Ch. 3.8 - Rank the following compounds in each set from most...Ch. 3.8 - In which solvent would cyclohexane have the lowest...Ch. 3.8 - Prob. 30PCh. 3.9 - Prob. 31PCh. 3.9 - a. Draw the three staggered conformations and the...Ch. 3.9 - a. Draw the most stable conformation of pentane...Ch. 3.11 - Draw 1,2,3,4,5,6-hexachlorocyclohexane with a. all...Ch. 3.12 - At any one time, would you expect there to be more...Ch. 3.13 - Prob. 36PCh. 3 - a. How many hydrogens does an alkane with 17...Ch. 3 - Prob. 2PCh. 3 - Draw a condensed structure and a skeletal...Ch. 3 - Prob. 39PCh. 3 - Prob. 40PCh. 3 - Which of the following represents a cis isomer?Ch. 3 - a. How many primary carbons does each of the...Ch. 3 - Name the following amines and tell whether they...Ch. 3 - Which of the following conformers of isobutyl...Ch. 3 - What is each compounds name? a. CH3CH2CH2OCH2CH3Ch. 3 - Draw the structural formula for an alkane that has...Ch. 3 - Which has a. the higher boiling point:...Ch. 3 - Ansaid and Motrin belong to the group of drugs...Ch. 3 - A student was given the structural formulas of...Ch. 3 - Which of the following conformers has the highest...Ch. 3 - Prob. 51PCh. 3 - Draw skeletal structures for the following: a....Ch. 3 - Prob. 53PCh. 3 - For rotation about the C-3 8 C-4 bond of...Ch. 3 - Prob. 55PCh. 3 - What is each compounds systematic name?Ch. 3 - Draw the two chair conformers for each of the...Ch. 3 - Draw the nine constitutional isomers with...Ch. 3 - Prob. 59PCh. 3 - Prob. 60PCh. 3 - Prob. 61PCh. 3 - Using Newman projections, draw the most stable...Ch. 3 - For each of the following disubstituted...Ch. 3 - Prob. 64PCh. 3 - Prob. 65PCh. 3 - What is each compounds systematic name?Ch. 3 - Prob. 67PCh. 3 - Bromine is a larger atom than chlorine, but the...Ch. 3 - Prob. 69PCh. 3 - Prob. 70PCh. 3 - a. Draw a potential energy diagram for rotation...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 9. Identify the relationship between the following pair of molecules? CI A. Same molecule. B. constitutional isomers. C. enantiomers. D. diastereomers E. different compounds (not isomers) and 10. Identify the relationship between the following pair of molecules? OH HO and HO HO A. Same molecule. B. constitutional isomers. C. enantiomers. D. diastereomers E. not isomers (different compounds)arrow_forwardThe given compound, 2-methylbutane is having A. One primary, one secondary and three tertiary carbons B. Three secondary, one primary and one tertiary carbons C. Three primary, one secondary and one tertiary carbons D. One primary, three secondary and one tertiary carbonsarrow_forwardHow many hydrogens do the following compound have? C9H?NO, has one ring and three double bond a. 13 b. 16 c. 12 d. 11arrow_forward
- The dimethylcyclohexane with the structure shown below is: ÇH3 CH3 Select one: O a. a trans isomer with the CH3 groups in axial positions. O b. a cis isomer with the CH3 groups in equatorial positions. O c. a cis isomer with the CH3 groups in equatorial and axial positions. O d. a trans isomer with the CH3 groups in equatorial positions.arrow_forward13. How many tertiary carbons and secondary hydrogens does the compound shown below contain? A. 5 tertiary carbons and 10 secondary hydrogens. B. 5 tertiary carbons and 5 secondary hydrogens. C. 3 tertiary carbons and 10 secondary hydrogens. D. 3 tertiary carbons and 12 secondary hydrogens. oulaarrow_forward7. Which pair of compounds are isomers? A. C4H4 & C4H8 С. CН:СH2CHCHCIz & CHCH2CH-CH2CI В. СН:СH-CHОН & CH;СH2CООН D. CH,CH2COОCH3 & CH;COОСH-CHзarrow_forward
- Identify the picture. a. Aliphatic HC : Alkane b. Aromatic HC c. Aliphatic HC d. Aromatic HC: Alkenearrow_forwardtof How are the molecules below related to each other? (Hint: convert each to a partially condensed structual formula). CH3 Н. HY H₂C CH3 CH3 and Н. H Select one: O A. They are stereoisomers. H H HỌC CHI CHO CH3 OB. They are constitutional isomers. d They are identical compounds. They are different compounds, not isomers.. OD. O E. They are geometrical isomers. Why does acetone [(CH3)2C=0, dipole moment = 2.69 D] have a larger dipole moment than phosgene [Cl₂C=0, dipole moment 1.17 D]? Note: electronegativities C = 2.5, Cl = 3.2, 0 = 3.5, H = 2.2arrow_forwardWhy the answer is C.arrow_forward
- 6. Consider the structure of cis-1,2-dimethylcyclopropane and trans-1,2-dimethylcyclopropane A. Which compound is more stable? Explain. B. Which compound will release more heat upon combustion? Explain C. Based on the following interactions, predict the difference in energy between the cis and trans isomers. Each CH3 Each CH3 Each H Each H CH3 gauche interaction is worth 0.9 kcal/mol CH3 eclipsed interaction is worth 2.6 kcal/mol CH3 eclipsed interaction is worth 1.4 kcal/mol H eclipsed interaction is worth 1.0 kcal/molarrow_forwardIdentify the highest and lowest energy conformation. If multiple energy conformations are degenerate, choose only one. Et H. Et Et H. H. MeEt Lowest Energy Me Highest Energy Et H. Et H. Et H. H- H. MeEt Highest Energy Me Lowest Energy Et H. Me H. Me C H H H. Me MeEt Highest Energy Lowest Energy Et H. Me Me H. H H Ме MeEt Lowest Energy Highest Energy OA OB ODarrow_forward6. Which of the following is not a constitutional isomer of the others? A. B. C. LOH etsarn D. -OHarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Nomenclature: Crash Course Chemistry #44; Author: CrashCourse;https://www.youtube.com/watch?v=U7wavimfNFE;License: Standard YouTube License, CC-BY