
EP ESSENTIAL ORG.CHEM.-MOD.MASTERING
3rd Edition
ISBN: 9780133858501
Author: Bruice
Publisher: PEARSON CO
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 3, Problem 43P
Name the following
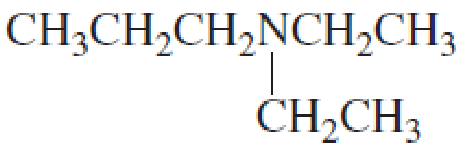
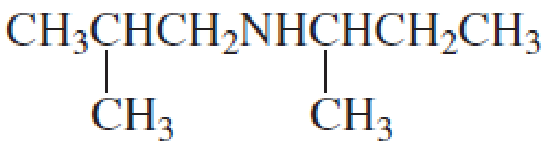
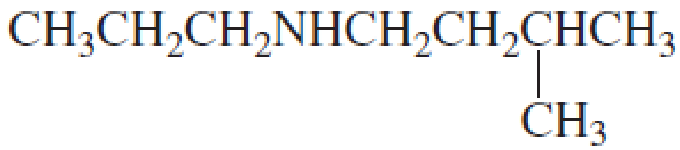
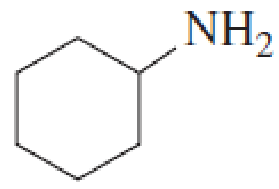
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Please help, draw and me the proper mechanisms.
Please help on part 2 not part 3. Please write in the proper reagents
Please help, draw and show me how to do it. Ignore the crossed out section.
Chapter 3 Solutions
EP ESSENTIAL ORG.CHEM.-MOD.MASTERING
Ch. 3.1 - Name each of the following:Ch. 3.1 - Draw the structures and name the four...Ch. 3.1 - Draw the structure for each of the following...Ch. 3.1 - Name the following compounds:Ch. 3.1 - Prob. 7PCh. 3.2 - What is each compounds systematic name?Ch. 3.2 - Prob. 11PCh. 3.3 - Prob. 12PCh. 3.3 - Convert the following condensed structures into...Ch. 3.3 - The molecular formula for ethyl alcohol (CH3CH2OH)...
Ch. 3.3 - Draw a condensed and a skeletal structure for the...Ch. 3.3 - What is each compounds systematic name?Ch. 3.4 - Prob. 17PCh. 3.4 - Give two names for each of the following alkyl...Ch. 3.5 - Are the following compounds primary, secondary, or...Ch. 3.5 - Name the following amines and tell whether they...Ch. 3.5 - Draw the structures and provide systematic names...Ch. 3.6 - Predict the approximate size of the following bond...Ch. 3.7 - What is the smallest straight-chain alkane that is...Ch. 3.7 - Prob. 24PCh. 3.7 - Prob. 25PCh. 3.7 - Prob. 26PCh. 3.7 - List the compounds in each set from highest...Ch. 3.8 - Rank the following compounds in each set from most...Ch. 3.8 - In which solvent would cyclohexane have the lowest...Ch. 3.8 - Prob. 30PCh. 3.9 - Prob. 31PCh. 3.9 - a. Draw the three staggered conformations and the...Ch. 3.9 - a. Draw the most stable conformation of pentane...Ch. 3.11 - Draw 1,2,3,4,5,6-hexachlorocyclohexane with a. all...Ch. 3.12 - At any one time, would you expect there to be more...Ch. 3.13 - Prob. 36PCh. 3 - a. How many hydrogens does an alkane with 17...Ch. 3 - Prob. 2PCh. 3 - Draw a condensed structure and a skeletal...Ch. 3 - Prob. 39PCh. 3 - Prob. 40PCh. 3 - Which of the following represents a cis isomer?Ch. 3 - a. How many primary carbons does each of the...Ch. 3 - Name the following amines and tell whether they...Ch. 3 - Which of the following conformers of isobutyl...Ch. 3 - What is each compounds name? a. CH3CH2CH2OCH2CH3Ch. 3 - Draw the structural formula for an alkane that has...Ch. 3 - Which has a. the higher boiling point:...Ch. 3 - Ansaid and Motrin belong to the group of drugs...Ch. 3 - A student was given the structural formulas of...Ch. 3 - Which of the following conformers has the highest...Ch. 3 - Prob. 51PCh. 3 - Draw skeletal structures for the following: a....Ch. 3 - Prob. 53PCh. 3 - For rotation about the C-3 8 C-4 bond of...Ch. 3 - Prob. 55PCh. 3 - What is each compounds systematic name?Ch. 3 - Draw the two chair conformers for each of the...Ch. 3 - Draw the nine constitutional isomers with...Ch. 3 - Prob. 59PCh. 3 - Prob. 60PCh. 3 - Prob. 61PCh. 3 - Using Newman projections, draw the most stable...Ch. 3 - For each of the following disubstituted...Ch. 3 - Prob. 64PCh. 3 - Prob. 65PCh. 3 - What is each compounds systematic name?Ch. 3 - Prob. 67PCh. 3 - Bromine is a larger atom than chlorine, but the...Ch. 3 - Prob. 69PCh. 3 - Prob. 70PCh. 3 - a. Draw a potential energy diagram for rotation...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning


Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax
Lipids - Fatty Acids, Triglycerides, Phospholipids, Terpenes, Waxes, Eicosanoids; Author: The Organic Chemistry Tutor;https://www.youtube.com/watch?v=7dmoH5dAvpY;License: Standard YouTube License, CC-BY