
EP ESSENTIAL ORG.CHEM.-MOD.MASTERING
3rd Edition
ISBN: 9780133858501
Author: Bruice
Publisher: PEARSON CO
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 3, Problem 42P
- a. How many primary carbons does each of the following compounds have?
- b. How many secondary carbons does each one have?
- c. How many tertiary carbons does each one have?
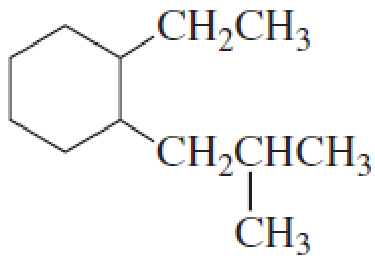
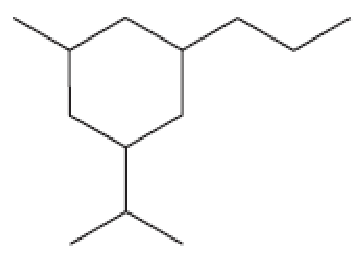
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Reason whether it is possible to determine changes in the Galvani potential difference at the metal-solution interface.
Obtain the standard potential at 25°C of the Cu* I Cu | Pt electrode
from the standard potentials E°
Cu²+/Cu
= 0.341 V and E
Cu²+ /Cu+
= 0.153 V.
In electrochemistry, briefly describe the Galvani potential, the Volta potential, and the surface potential. Differentiate between them.
Chapter 3 Solutions
EP ESSENTIAL ORG.CHEM.-MOD.MASTERING
Ch. 3.1 - Name each of the following:Ch. 3.1 - Draw the structures and name the four...Ch. 3.1 - Draw the structure for each of the following...Ch. 3.1 - Name the following compounds:Ch. 3.1 - Prob. 7PCh. 3.2 - What is each compounds systematic name?Ch. 3.2 - Prob. 11PCh. 3.3 - Prob. 12PCh. 3.3 - Convert the following condensed structures into...Ch. 3.3 - The molecular formula for ethyl alcohol (CH3CH2OH)...
Ch. 3.3 - Draw a condensed and a skeletal structure for the...Ch. 3.3 - What is each compounds systematic name?Ch. 3.4 - Prob. 17PCh. 3.4 - Give two names for each of the following alkyl...Ch. 3.5 - Are the following compounds primary, secondary, or...Ch. 3.5 - Name the following amines and tell whether they...Ch. 3.5 - Draw the structures and provide systematic names...Ch. 3.6 - Predict the approximate size of the following bond...Ch. 3.7 - What is the smallest straight-chain alkane that is...Ch. 3.7 - Prob. 24PCh. 3.7 - Prob. 25PCh. 3.7 - Prob. 26PCh. 3.7 - List the compounds in each set from highest...Ch. 3.8 - Rank the following compounds in each set from most...Ch. 3.8 - In which solvent would cyclohexane have the lowest...Ch. 3.8 - Prob. 30PCh. 3.9 - Prob. 31PCh. 3.9 - a. Draw the three staggered conformations and the...Ch. 3.9 - a. Draw the most stable conformation of pentane...Ch. 3.11 - Draw 1,2,3,4,5,6-hexachlorocyclohexane with a. all...Ch. 3.12 - At any one time, would you expect there to be more...Ch. 3.13 - Prob. 36PCh. 3 - a. How many hydrogens does an alkane with 17...Ch. 3 - Prob. 2PCh. 3 - Draw a condensed structure and a skeletal...Ch. 3 - Prob. 39PCh. 3 - Prob. 40PCh. 3 - Which of the following represents a cis isomer?Ch. 3 - a. How many primary carbons does each of the...Ch. 3 - Name the following amines and tell whether they...Ch. 3 - Which of the following conformers of isobutyl...Ch. 3 - What is each compounds name? a. CH3CH2CH2OCH2CH3Ch. 3 - Draw the structural formula for an alkane that has...Ch. 3 - Which has a. the higher boiling point:...Ch. 3 - Ansaid and Motrin belong to the group of drugs...Ch. 3 - A student was given the structural formulas of...Ch. 3 - Which of the following conformers has the highest...Ch. 3 - Prob. 51PCh. 3 - Draw skeletal structures for the following: a....Ch. 3 - Prob. 53PCh. 3 - For rotation about the C-3 8 C-4 bond of...Ch. 3 - Prob. 55PCh. 3 - What is each compounds systematic name?Ch. 3 - Draw the two chair conformers for each of the...Ch. 3 - Draw the nine constitutional isomers with...Ch. 3 - Prob. 59PCh. 3 - Prob. 60PCh. 3 - Prob. 61PCh. 3 - Using Newman projections, draw the most stable...Ch. 3 - For each of the following disubstituted...Ch. 3 - Prob. 64PCh. 3 - Prob. 65PCh. 3 - What is each compounds systematic name?Ch. 3 - Prob. 67PCh. 3 - Bromine is a larger atom than chlorine, but the...Ch. 3 - Prob. 69PCh. 3 - Prob. 70PCh. 3 - a. Draw a potential energy diagram for rotation...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What substances can neutralize, complex or adsorb and absorb both HF and CF carbonyl fluoride and hydrogen fluoride and intermediate formation of thermal decomposition of fluorinated inorganic compounds either due to hydrolysis and hygroscopic reactions. What is the known chemistry of these reactions and mechanisms.arrow_forwardBriefly differentiate between chemical potential and electrochemical potential.arrow_forwardAccording to open access forums ionic antimony Sb (111) can be reduced to elemental Sb (0) in solution and in macromolecules like condensation polymers polyethylene terephthalate (PET) causing greying of the polymer matrix. It has been connected to thermal degradation of the polymer during processing to the formation of thermally unstable EG ethyleen glycol that forms at various temperatures formic acid, formaldehyde, acetaldehyde and much more depending on temperature. I need to know what organics are more powerful reducing agents and at what concentration (relative) to each organic will initiate this reduction. Furthermore, is the pH dependant ? Are other trace elements in the plastic also a cause of concern e.g. aluminum from aluminum chloride (lewis acid). Therefore, the ultimate solution should include a means to inhibit reduction of ionic antimony and will the same solution comply with cobalt impurities from ionic cobalt? Some PET have combinations of catalyst and their residues…arrow_forward
- From a pH standpoint is the reduction of ionic Antimony Sb (111) to elemental Sb (0) occur more readily by acidic species acting as reducing agents or basic substances? I want to inhibit this reduction of ionic to elemental. Suggestions and directions!arrow_forwardObtain the standard potential at 25°C of the Cu* I Cu | Pt electrode from the standard potentials E° Cu²+/Cu = 0.341 V and E Cu²+ /Cu+ = 0.153 V.arrow_forwardState two variables on which the transport number in electrochemistry depends.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Nomenclature: Crash Course Chemistry #44; Author: CrashCourse;https://www.youtube.com/watch?v=U7wavimfNFE;License: Standard YouTube License, CC-BY