
Chemistry for Changing Times
14th Edition
ISBN: 9780134212777
Author: John W. Hill; Terry W. McCreary
Publisher: Pearson Education (US)
expand_more
expand_more
format_list_bulleted
Question
Chapter 3, Problem 34P
a.
Interpretation Introduction
Interpretation:
Whether electronic configuration: 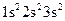 represents an atom in the ground state or in a possible excited state or is incorrect should be determined.
represents an atom in the ground state or in a possible excited state or is incorrect should be determined.
Concept introduction:
- The arrangement of electrons in an atom by a superscript, in each sublevel, is known as electron configuration. For example, the d block elements have
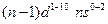 electronic configuration
electronic configuration - When one or more electrons are promoted to a higher energy level of electron configuration is known as the excited state.
b.
Interpretation Introduction
Interpretation:
Whether electronic configuration: 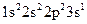 represents an atom in the ground state or in a possible excited state or is incorrect should be determined.
represents an atom in the ground state or in a possible excited state or is incorrect should be determined.
Concept introduction:
- The arrangement of electrons in an atom by a superscript, in each sublevel, is known as electron configuration. For example, the d block elements have
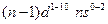 electronic configuration
electronic configuration - When one or more electrons are promoted to a higher energy level of electron configuration is known as the excited state.
c.
Interpretation Introduction
Interpretation:
Whether electronic configuration: 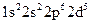 represents an atom in the ground state or in a possible excited state or is incorrect should be determined.
represents an atom in the ground state or in a possible excited state or is incorrect should be determined.
Concept introduction:
- The arrangement of electrons in an atom by a superscript, in each sublevel, is known as electron configuration. For example, the d block elements have
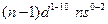 electronic configuration
electronic configuration - When one or more electrons are promoted to a higher energy level of electron configuration is known as the excited state.
d.
Interpretation Introduction
Interpretation:
Whether electronic configuration: 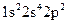 represents an atom in the ground state or in a possible excited state or is incorrect should be determined.
represents an atom in the ground state or in a possible excited state or is incorrect should be determined.
Concept introduction:
- The arrangement of electrons in an atom by a superscript, in each sublevel, is known as electron configuration. For example, the d block elements have
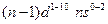 electronic configuration
electronic configuration - When one or more electrons are promoted to a higher energy level of electron configuration is known as the excited state.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
NH3 decomposes through an equilibrium reaction between NH3, H2, and N2. Only one of the options is correct:(A). The mechanism of the NH3 decomposition reaction must necessarily involve the collision of two NH3 molecules to induce a rearrangement of the atoms in this molecule.(B). The molecular weight of the NH3 decomposition reaction is 2 since two NH3 molecules must collide.(C). The rate of the NH3 decomposition reaction must be greater than that of NH3 synthesis, since the former requires two molecules to collide and the latter, four.(D). The NH3 decomposition reaction cannot occur in a single step.
Given the equilibrium A2 + B2 ⇌ 2 AB where k1 is the rate coefficient of the forward reaction and k-1 is the rate coefficient of the reverse reaction, with the forward reaction being first-order in A2 and B2, and the reverse reaction being second-order in AB. Equilibrium will be reached later if the relative values of the constants are:(A) k1 high and k-1 high(B) k1 high and k-1 low(C) k1 low and k-1 high(D) k1 low and k-1 low
A 2-step reaction has the following mechanism:
|
1. (fast) R2
R+R
2. (slow) R+Q
K₂ P
k_1
What series does it have?
(A). v=
-
= (k + k1 − k-1)[R2][Q]
(B). v=-k₁[R₂] + k₁[R]² - k₂[R][Q]
(C). v=k₂[R]²[Q]²
(D). v = k[R₂]1/2[Q]
Chapter 3 Solutions
Chemistry for Changing Times
Ch. 3 - What did each of the following scientists...Ch. 3 - What is radioactivity? How did the discovery of...Ch. 3 - How are X-rays and gamma rays similar? How are...Ch. 3 - How was Goldstein's experiment different from...Ch. 3 - In Rutherford's model of the atom, where are the...Ch. 3 - Prob. 6RQCh. 3 - Prob. 7RQCh. 3 - Prob. 8RQCh. 3 - Prob. 9RQCh. 3 - 10. What are the symbol, name, and atomic number...
Ch. 3 - What are the symbol, name, and atomic number of...Ch. 3 - Prob. 12RQCh. 3 - Prob. 13RQCh. 3 - 14. How did Bohr and Schrodinger refine the model...Ch. 3 - Prob. 15RQCh. 3 - 16. Use the periodic table to determine the number...Ch. 3 - Prob. 17RQCh. 3 - Prob. 18PCh. 3 - Prob. 19PCh. 3 - Prob. 20PCh. 3 - Prob. 21PCh. 3 - A neutral atom with 9 protons will have how many...Ch. 3 - Give the symbol and name for (a) an isotope with a...Ch. 3 - Fill in the table: Element Mass Number Number of...Ch. 3 - Prob. 25PCh. 3 - Prob. 26PCh. 3 - Prob. 27PCh. 3 - Prob. 28PCh. 3 - Prob. 29PCh. 3 - Prob. 30PCh. 3 - Without referring to the periodic table, give the...Ch. 3 - Prob. 32PCh. 3 - 33. Indicate whether each electron configuration...Ch. 3 - Prob. 34PCh. 3 - Prob. 35PCh. 3 - Prob. 36PCh. 3 - Prob. 37PCh. 3 - Referring only to the periodic table, tell how the...Ch. 3 - Prob. 39PCh. 3 - Use the following list of elements to answer...Ch. 3 - Prob. 41PCh. 3 - Prob. 42PCh. 3 - Prob. 43PCh. 3 - Use the following list of elements to answer...Ch. 3 - Prob. 45APCh. 3 - Prob. 46APCh. 3 - Prob. 47APCh. 3 - Look again at Figure 3.11. A patient is found to...Ch. 3 - Refer to Figure 3.16 and write the electron...Ch. 3 - Prob. 50APCh. 3 - Prob. 51APCh. 3 - Prob. 52APCh. 3 - Prob. 53APCh. 3 - Prob. 54APCh. 3 - 55. Which of the following is not a benefit of...Ch. 3 - Prob. 56APCh. 3 - Prob. 3.1CTECh. 3 - Prob. 3.2CTECh. 3 - Prob. 3.3CTECh. 3 - Prob. 3.4CTECh. 3 - Prepare a PowerPoint, poster, or other...Ch. 3 - Prob. 2CGPCh. 3 - Prob. 3CGPCh. 3 - Prepare a PowerPoint, poster, or other...Ch. 3 - Prob. 1CHQCh. 3 - Materials requried
Colorflame birthday candles...Ch. 3 - Prob. 3CHQCh. 3 - Prob. 4CHQCh. 3 - Prob. 5CHQ
Knowledge Booster
Similar questions
- Steps and explanations. Also provide, if possible, ways to adress this kind of problems in general.arrow_forwardSteps and explanations. Also provide, if possible, ways to adress this kind of problems in general.arrow_forwardSteps and explanations. Also provide, if possible, ways to adress this kind of problems in general.arrow_forward
- Steps and explanations. Also provide, if possible, ways to adress this kind of problems in general.arrow_forwardSteps and explanations. Also provide, if possible, ways to adress this kind of problems in general.arrow_forwardSteps and explanations. Also provide, if possible, ways to adress this kind of problems in general.arrow_forward
- Label the α and ẞ carbons in each alkyl halide. Draw all possible elimination products formed when each alkyl halide is treated with K-OC(CH3), b. ان Brarrow_forwardSuppose a reaction has the following mechanism:A + B → C + D C + C → F F + B → A + A + GIt is known that C is a reaction intermediate. Of the following options, indicate which are true:1. The overall reaction could be 3B → 2D + G.2. A could be a catalyst.3. C is the only intermediate that can exist.arrow_forwardSteps and explanations. Also provide, if possible, ways to adress this kind of problems in general.arrow_forward
- Steps and explanations. Also provide, if possible, ways to adress this kind of problems in general.arrow_forwardSteps and explanations. Also provide, if possible, ways to adress this kind of problems in general.arrow_forwardSteps and explanations. Also provide, if possible, ways to adress this kind of problems in general.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY