
Fundamentals Of Thermodynamics
10th Edition
ISBN: 9781119494966
Author: Borgnakke, C. (claus), Sonntag, Richard Edwin, Author.
Publisher: Wiley,
expand_more
expand_more
format_list_bulleted
Textbook Question
thumb_up100%
Chapter 3, Problem 3.47P
A radiant heat lamp is a rod, 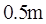 tong and
tong and 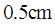 in diameter through which
in diameter through which 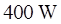 of electric energy is deposited Assume that the surface has an emissivity of 0.9 and neglect incoming radiation. What will the rod surface temperature be?
of electric energy is deposited Assume that the surface has an emissivity of 0.9 and neglect incoming radiation. What will the rod surface temperature be?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Find the equivalent mass of the rocker arm assembly with respect to the x coordinate.
k₁
mi
m2
k₁
2. Figure below shows a U-tube manometer open at both ends and containing a column of liquid
mercury of length l and specific weight y. Considering a small displacement x of the manometer
meniscus from its equilibrium position (or datum), determine the equivalent spring constant associated
with the restoring force.
Datum
Area, A
1. The consequences of a head-on collision of two automobiles can be studied by considering the
impact of the automobile on a barrier, as shown in figure below. Construct a mathematical model (i.e.,
draw the diagram) by considering the masses of the automobile body, engine, transmission, and
suspension and the elasticity of the bumpers, radiator, sheet metal body, driveline, and engine
mounts.
Chapter 3 Solutions
Fundamentals Of Thermodynamics
Ch. 3 - What is 1cal in SI units and what is the name...Ch. 3 - A car engine is rated at 110kW . What is the power...Ch. 3 - Why do we write E or E2E1 , whereas we write 1Q2...Ch. 3 - If a process in a control mass increases energy...Ch. 3 - Assume a physical setup as in Fig. P3.6(a). We now...Ch. 3 - Figure P3.6 shows three physical situations. Show...Ch. 3 - For the indicated physical setup in (a), (b), and...Ch. 3 - Assume the physical situation in Fig. P3.6b; what...Ch. 3 - Figure P3.9 shows three physical situations; show...Ch. 3 - That can you say about the beginning state of the...
Ch. 3 - A thermopane window traps some gas between the two...Ch. 3 - Prob. 3.12PCh. 3 - Prob. 3.13PCh. 3 - The electric bill is calculating usage in kWh....Ch. 3 - Prob. 3.15PCh. 3 - Prob. 3.16PCh. 3 - Prob. 3.17PCh. 3 - You heat a gas 10K at P=C . Which one in Table A.5...Ch. 3 - You mix 20C water with 50C water in an open...Ch. 3 - A piston motion moves a 25kg hammerhead vertically...Ch. 3 - A pump pushes 1000m3 of liquid water at 15C up to...Ch. 3 - A 1200kg car accelerates from zero to 100km/h over...Ch. 3 - A hydraulic hoist raises a 1750kg car 1.8m in an...Ch. 3 - Prob. 3.24PCh. 3 - A hydraulic cylinder of area 0.01m2 must push a...Ch. 3 - A hydraulic cylinder has a piston cross-sectional...Ch. 3 - A bulldozer pushes 800kg of din l00m with a force...Ch. 3 - Two hydraulic cylinders maintain a pressure of...Ch. 3 - A motor delivers 50hp on a drive shaft at 1800rpm...Ch. 3 - Solve Problem 3.24, but assume that the steam...Ch. 3 - The R4l0A in Problem 3.1 2c is at 1000kPa,50C with...Ch. 3 - A 400L tank, A (see Fig. P3.32), contains argon...Ch. 3 - A piston/cylinder assembly contains 2kg of liquid...Ch. 3 - Heat transfer to a 1.5kg block of ice at -10C...Ch. 3 - A cylinder fitted with a frictionless piston...Ch. 3 - A piston cylinder contains 2kg of water at 20C...Ch. 3 - A nitrogen gas goes through a polytropic process...Ch. 3 - Helium gas expands from 125kPa,350K and 0.25m3 to...Ch. 3 - A balloon behaves so that the pressure is P=C2V1/3...Ch. 3 - A 15cm thick concrete wall, k=1.28W/mK , has a...Ch. 3 - The brake shoe and steel drum of a car...Ch. 3 - Prob. 3.42PCh. 3 - A power plant condenser (heat exchanger) transfers...Ch. 3 - Prob. 3.44PCh. 3 - A steel Pot, with conductivity of 15W/m and a 50mm...Ch. 3 - A wall surface on a house is 30C with an...Ch. 3 - A radiant heat lamp is a rod, tong and in diameter...Ch. 3 - A radiant beating lamp has a surface temperature...Ch. 3 - Determine the phase of the following substances...Ch. 3 - Find the phase and the missing properties of P, T,...Ch. 3 - Indicate the location of the four states in...Ch. 3 - Find the missing property of P, T, y, u, h, and x...Ch. 3 - Find the missing properties for carbon dioxide at...Ch. 3 - Find the missing property of P, T, y, u, h, and x...Ch. 3 - Saturated liquid water at 20C is compressed to a...Ch. 3 - Consider a steel bottle as a CV. It contains...Ch. 3 - A piston cylinder contains water with quality 75...Ch. 3 - Problem 3.137 and write the left-hand side...Ch. 3 - Saturated vapor R410A at 0C in a rigid tank is...Ch. 3 - A constant-pressure piston/cylinder assembly...Ch. 3 - A container is split in two equal volumes by a...Ch. 3 - A cylinder fined with a frictionless piston...Ch. 3 - A piston/cylinder contains 1.5kg of water at...Ch. 3 - Ammonia (0.5kg) in a piston cy1tnde at 200kPa,10C...Ch. 3 - A water-filled reactor with a volume of 1m3 is at...Ch. 3 - A rigid 1kg steel tank holds 0.75kg ammonia at 70C...Ch. 3 - Prob. 3.68PCh. 3 - A piston/cylinder arrangement with a linear spring...Ch. 3 - Prob. 3.70PCh. 3 - Assume the same setup as in Problem 3.66. but the...Ch. 3 - A rigid steel tank contains 0.5kgR410A at 0°C with...Ch. 3 - Redo the previous problem when you also consider...Ch. 3 - Prob. 3.74PCh. 3 - Supetheated refrigerant R-134a at 20°C and 100 kPa...Ch. 3 - In a sink, 5 L of water at 70°C is combined with 1...Ch. 3 - Prob. 3.77PCh. 3 - A copper block of volume 1 L s heat treated at...Ch. 3 - A car with mass 1275 kg is driven at 60 km h when...Ch. 3 - A piston cylinder (0.5 kg steel altogether)...Ch. 3 - An engine, shown in Fig P3.81, consists of a 100kg...Ch. 3 - Use the ideal gas air A.7 to evaluate the specific...Ch. 3 - Estimate the constant specific heats for R-134a...Ch. 3 - Find the change in u for carbon dioxide between...Ch. 3 - Nitrogen at 300 K. 3 MPa is heated to 500 K Find...Ch. 3 - Repeat Problem 3.84 for nitrogen gas.Ch. 3 - Find the change in enthalpy for carbon dioxide...Ch. 3 - Water at 20°C and 100 kPa is brought to l00 kPa...Ch. 3 - Prob. 3.89PCh. 3 - A rigid container has 2 kg of oxygen gas at l00...Ch. 3 - Air (3kg) is in a piston cylinder similar to...Ch. 3 - A 10-m-high cylinder. with a cross-sectional area...Ch. 3 - A cylinder with a piston restrained by a linear...Ch. 3 - A constant pressure container is filled with 1 kg...Ch. 3 - A spring-loaded piston cylinder contains 1.5kg of...Ch. 3 - An insulated cylinder is divided into two pans of...Ch. 3 - Helium gas expands from 125 kPa, 350 K and 0.25m3...Ch. 3 - A piston cylinder device contains 0.1 kg of air at...Ch. 3 - A gasoline engine has a piston/cylinder with 0.1...Ch. 3 - Solve the previous problem using Table A.7.Ch. 3 - A piston/cylinder has nitrogen gas at 750 K and...Ch. 3 - A piston/cylinder assembly has 1 kg of propane gas...Ch. 3 - A piston cylinder arrangement of initial volume...Ch. 3 - A piston/cylinder assembly in a car contains 0.2 L...Ch. 3 - Air goes through a polytropic process with n=1.3...Ch. 3 - Saturated vapor R410A at 10°C of mass 0.6 kg is in...Ch. 3 - A helium gas heated at constant volume from 100...Ch. 3 - A piston/cylinder shown in Fig. P3.108 contains...Ch. 3 - A piston/cylinder has water at 200 kPa, x=0.5 and...Ch. 3 - Ten kilograms of water in a piston/cylinder...Ch. 3 - Water in piston/cylinder (Fig. P3.111) is a...Ch. 3 - A setup lake the one in Fig P3.108 has the R-410A...Ch. 3 - The piston/cylinder inFig. P3.113 contains 0.1 kg...Ch. 3 - A piston/cylinder arrangement contains 5 kg of...Ch. 3 - A piston/cylinder setup similar to Problem 3.110...Ch. 3 - A piston/cylinder contains air at 1000 kPa, 800 K...Ch. 3 - Prob. 3.117PCh. 3 - A 100-hp car engine has a drive shaft rotating at...Ch. 3 - Prob. 3.119PCh. 3 - As fresh-poured concrete hardens, the chemical...Ch. 3 - A 1.2-kg pot of water at 20°C is put on a stove...Ch. 3 - A computer in a closed room of volume 200m3...Ch. 3 - A 500-W heater is used to melt 2 kg of solid ice...Ch. 3 - A 3-kg mass of nitrogen gas at 2000 K, V=C , cools...Ch. 3 - Electric power as volts times amperes (P=Vi) ....Ch. 3 - A copper wire of diameter 2 mm is 10m long and...Ch. 3 - A battery is well insulated while being charged by...Ch. 3 - A sheet of rubberis stretched out over a ring of...Ch. 3 - Assume a balloon material with a constant surface...Ch. 3 - A soap bubble has a surface tension of =3104N/cm...Ch. 3 - According to Table 3.4 residential buildings in US...Ch. 3 - total energy use in the US from Table 3.4 for 2011...Ch. 3 - A wind turbine with 20m diameter rotors spins at...Ch. 3 - Prob. 3.134PCh. 3 - A house is being designed to use a thick concrete...Ch. 3 - A solar pond with 20°C salt water, Cp=3.8kJ/kg-K...Ch. 3 - Prob. 3.137PCh. 3 - A rigidtank is divided into tworooms,both...Ch. 3 - A piston/cylinder has a water volume separated in...Ch. 3 - The cylinder volume below the constant loaded...Ch. 3 - Air in tank B is at 200 kPa, 280 K and mass 1 kg....Ch. 3 - A piston/cylinder setup (Fig P3.110) contains 1 kg...Ch. 3 - Two kilograms of water is contained in a...Ch. 3 - A piston cylinder has 0.1 kg water at x=0.5 ,...Ch. 3 - A piston/cylinder arrangement has the piston...Ch. 3 - A vertical/cylinder (Fig. P3.146) has a 61.18-kg...Ch. 3 - Water in a piston/cylinder, similar to Fig P3.110,...Ch. 3 - A rigid container has two rooms filled with water,...Ch. 3 - Prob. 3.149PCh. 3 - A piston/cylinder setup, similar to Fig. P3.143,...Ch. 3 - A spherical balloon contains 2 kg of R-410A at 0°C...Ch. 3 - Prob. 3.152PCh. 3 - Prob. 3.153EPCh. 3 - Work as Fx has units of lbf ft. What is that in...Ch. 3 - Work in the expression in Eq. 3.18 or Eq. 3.22...Ch. 3 - Prob. 3.156EPCh. 3 - Prob. 3.157EPCh. 3 - You heat a gas 20 R at P=C . Which gas in Table...Ch. 3 - A piston motion moves a 50-lbm hammerhead...Ch. 3 - A pump pushes 35000ft3 of liquid water at 60 F up...Ch. 3 - Prob. 3.161EPCh. 3 - Prob. 3.162EPCh. 3 - Prob. 3.163EPCh. 3 - A car with tires of outer radius 12 in. drives...Ch. 3 - The R-410A in Problem 3.9(c) is at 150 psia, 120 F...Ch. 3 - Prob. 3.166EPCh. 3 - A nitrogen gas goes through apolytropic process...Ch. 3 - Find the rate of conduction heat transfer per unit...Ch. 3 - The sun shines on a 1500-ft2 road surface so that...Ch. 3 - Find the missing properties and give the phase of...Ch. 3 - Find the missing properties among (P,T,v,u,h)...Ch. 3 - Find the missing properties among (P,T,v,u,h)...Ch. 3 - Saturated vapor R-410A at 60 F in a rigid tank is...Ch. 3 - A containeris split in two equal volumes by a...Ch. 3 - Saturated vapor R-410A at 200 psia in a...Ch. 3 - A cylinder fitted with a frictionless piston...Ch. 3 - Prob. 3.177EPCh. 3 - A water-filled reactor with a volume of 50ft3 is...Ch. 3 - Prob. 3.179EPCh. 3 - A piston/cylinder arrangement with a linear spring...Ch. 3 - Prob. 3.181EPCh. 3 - Prob. 3.182EPCh. 3 - Prob. 3.183EPCh. 3 - Prob. 3.184EPCh. 3 - Prob. 3.185EPCh. 3 - A closed rigid container is filled with 3 lbm...Ch. 3 - An insulated cylinder is divided into two parts of...Ch. 3 - Helium gas expands from 20 psia, 600 R, and 9ft3...Ch. 3 - Prob. 3.189EPCh. 3 - A cylinder fitted with a frictionless piston...Ch. 3 - Prob. 3.191EPCh. 3 - A piston/cylinder contains air at 150 psia, 1400 R...Ch. 3 - A piston/cylinder has 2 lbm of R-134a at state 1...Ch. 3 - A force of 300 lbf moves a truck at a speed of 40...Ch. 3 - Prob. 3.195EPCh. 3 - Water is in a piston/cylinder maintaining constant...Ch. 3 - A mass of 6 lbm nitrogen gas at 3600 R, V=C ,...Ch. 3 - Prob. 3.198EPCh. 3 - Ammonia is contained in a sealed, rigid tank at 30...Ch. 3 - Water in a piston/cylinder, similar to Fig....
Additional Engineering Textbook Solutions
Find more solutions based on key concepts
Stock Profit The profit from the sale of a stock can be calculated as follows: Profit=((NSSP)SC)((NSPP)+PC) whe...
Starting Out with Java: From Control Structures through Data Structures (4th Edition) (What's New in Computer Science)
In the following exercises, write a program to carry out the task. The program should use variables for each of...
Introduction To Programming Using Visual Basic (11th Edition)
For the circuit shown, use the node-voltage method to find v1, v2, and i1.
How much power is delivered to the c...
Electric Circuits. (11th Edition)
CONCEPT QUESTIONS
15.CQ3 The ball rolls without slipping on the fixed surface as shown. What is the direction ...
Vector Mechanics for Engineers: Statics and Dynamics
What is the output of the following (when embedded in a complete program)? int n = 1; do cout n ; while (n++...
Problem Solving with C++ (10th Edition)
Consider the boolean expression (2 5) (x 100)). Why is this expression probably not what the programmer inte...
Java: An Introduction to Problem Solving and Programming (8th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Similar questions
- 3.) 15.40 – Collar B moves up at constant velocity vB = 1.5 m/s. Rod AB has length = 1.2 m. The incline is at angle = 25°. Compute an expression for the angular velocity of rod AB, ė and the velocity of end A of the rod (✓✓) as a function of v₂,1,0,0. Then compute numerical answers for ȧ & y_ with 0 = 50°.arrow_forward2.) 15.12 The assembly shown consists of the straight rod ABC which passes through and is welded to the grectangular plate DEFH. The assembly rotates about the axis AC with a constant angular velocity of 9 rad/s. Knowing that the motion when viewed from C is counterclockwise, determine the velocity and acceleration of corner F.arrow_forward500 Q3: The attachment shown in Fig.3 is made of 1040 HR. The static force is 30 kN. Specify the weldment (give the pattern, electrode number, type of weld, length of weld, and leg size). Fig. 3 All dimension in mm 30 kN 100 (10 Marks)arrow_forward
- (read image) (answer given)arrow_forwardA cylinder and a disk are used as pulleys, as shown in the figure. Using the data given in the figure, if a body of mass m = 3 kg is released from rest after falling a height h 1.5 m, find: a) The velocity of the body. b) The angular velocity of the disk. c) The number of revolutions the cylinder has made. T₁ F Rd = 0.2 m md = 2 kg T T₂1 Rc = 0.4 m mc = 5 kg ☐ m = 3 kgarrow_forward(read image) (answer given)arrow_forward
- 11-5. Compute all the dimensional changes for the steel bar when subjected to the loads shown. The proportional limit of the steel is 230 MPa. 265 kN 100 mm 600 kN 25 mm thickness X Z 600 kN 450 mm E=207×103 MPa; μ= 0.25 265 kNarrow_forwardT₁ F Rd = 0.2 m md = 2 kg T₂ Tz1 Rc = 0.4 m mc = 5 kg m = 3 kgarrow_forward2. Find a basis of solutions by the Frobenius method. Try to identify the series as expansions of known functions. (x + 2)²y" + (x + 2)y' - y = 0 ; Hint: Let: z = x+2arrow_forward
- 1. Find a power series solution in powers of x. y" - y' + x²y = 0arrow_forward3. Find a basis of solutions by the Frobenius method. Try to identify the series as expansions of known functions. 8x2y" +10xy' + (x 1)y = 0 -arrow_forwardHello I was going over the solution for this probem and I'm a bit confused on the last part. Can you please explain to me 1^4 was used for the Co of the tubular cross section? Thank you!arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Principles of Heat Transfer (Activate Learning wi...Mechanical EngineeringISBN:9781305387102Author:Kreith, Frank; Manglik, Raj M.Publisher:Cengage Learning
Principles of Heat Transfer (Activate Learning wi...Mechanical EngineeringISBN:9781305387102Author:Kreith, Frank; Manglik, Raj M.Publisher:Cengage Learning

Principles of Heat Transfer (Activate Learning wi...
Mechanical Engineering
ISBN:9781305387102
Author:Kreith, Frank; Manglik, Raj M.
Publisher:Cengage Learning
Physics 33 - Fluid Statics (1 of 10) Pressure in a Fluid; Author: Michel van Biezen;https://www.youtube.com/watch?v=mzjlAla3H1Q;License: Standard YouTube License, CC-BY