
Fundamentals Of Thermodynamics
10th Edition
ISBN: 9781119494966
Author: Borgnakke, C. (claus), Sonntag, Richard Edwin, Author.
Publisher: Wiley,
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 3, Problem 3.6P
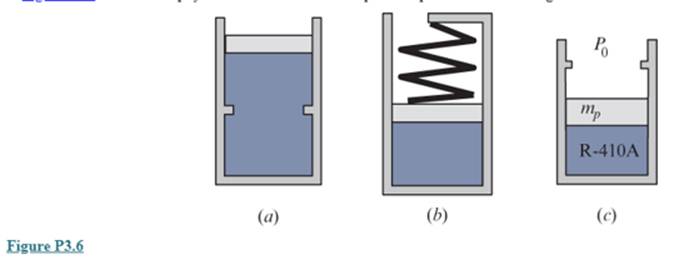
Figure P3.6 shows three physical situations. Show the possible process in a
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
!
Required information
A one-shell-pass and eight-tube-passes heat exchanger is used to heat glycerin (cp=0.60 Btu/lbm.°F) from 80°F to 140°F
by hot water (Cp = 1.0 Btu/lbm-°F) that enters the thin-walled 0.5-in-diameter tubes at 175°F and leaves at 120°F. The total
length of the tubes in the heat exchanger is 400 ft. The convection heat transfer coefficient is 4 Btu/h-ft²°F on the glycerin
(shell) side and 70 Btu/h-ft²°F on the water (tube) side.
NOTE: This is a multi-part question. Once an answer is submitted, you will be unable to return to this part.
Determine the rate of heat transfer in the heat exchanger before any fouling occurs.
Correction factor F
1.0
10
0.9
0.8
R=4.0 3.0 2.0.15 1.0 0.8.0.6 0.4 0.2
0.7
0.6
R=
T1-T2
12-11
0.5
12-11
0 0.1 0.2 0.3 0.4 0.5 0.6 0.7 0.8 0.9 1.0
(a) One-shell pass and 2, 4, 6, etc. (any multiple of 2), tube passes
P=
T₁-11
The rate of heat transfer in the heat exchanger is
Btu/h.
!
Required information
Air at 25°C (cp=1006 J/kg.K) is to be heated to 58°C by hot oil at 80°C (cp = 2150 J/kg.K) in a cross-flow heat exchanger
with air mixed and oil unmixed. The product of heat transfer surface area and the overall heat transfer coefficient is 750
W/K and the mass flow rate of air is twice that of oil.
NOTE: This is a multi-part question. Once an answer is submitted, you will be unable to return to this part.
Air
Oil
80°C
Determine the effectiveness of the heat exchanger.
In an industrial facility, a counter-flow double-pipe heat exchanger uses superheated steam at a temperature of 155°C to
heat feed water at 30°C. The superheated steam experiences a temperature drop of 70°C as it exits the heat exchanger.
The water to be heated flows through the heat exchanger tube of negligible thickness at a constant rate of 3.47 kg/s. The
convective heat transfer coefficient on the superheated steam and water side is 850 W/m²K and 1250 W/m²K,
respectively. To account for the fouling due to chemical impurities that might be present in the feed water, assume
a fouling factor of 0.00015 m²-K/W for the water side.
The specific heat of water is determined at an average temperature of (30 +70)°C/2 = 50°C and is taken to be
J/kg.K.
Cp=
4181
Water
Steam
What would be the required heat exchanger area in case of parallel-flow arrangement?
The required heat exchanger area in case of parallel-flow arrangement is
1m².
Chapter 3 Solutions
Fundamentals Of Thermodynamics
Ch. 3 - What is 1cal in SI units and what is the name...Ch. 3 - A car engine is rated at 110kW . What is the power...Ch. 3 - Why do we write E or E2E1 , whereas we write 1Q2...Ch. 3 - If a process in a control mass increases energy...Ch. 3 - Assume a physical setup as in Fig. P3.6(a). We now...Ch. 3 - Figure P3.6 shows three physical situations. Show...Ch. 3 - For the indicated physical setup in (a), (b), and...Ch. 3 - Assume the physical situation in Fig. P3.6b; what...Ch. 3 - Figure P3.9 shows three physical situations; show...Ch. 3 - That can you say about the beginning state of the...
Ch. 3 - A thermopane window traps some gas between the two...Ch. 3 - Prob. 3.12PCh. 3 - Prob. 3.13PCh. 3 - The electric bill is calculating usage in kWh....Ch. 3 - Prob. 3.15PCh. 3 - Prob. 3.16PCh. 3 - Prob. 3.17PCh. 3 - You heat a gas 10K at P=C . Which one in Table A.5...Ch. 3 - You mix 20C water with 50C water in an open...Ch. 3 - A piston motion moves a 25kg hammerhead vertically...Ch. 3 - A pump pushes 1000m3 of liquid water at 15C up to...Ch. 3 - A 1200kg car accelerates from zero to 100km/h over...Ch. 3 - A hydraulic hoist raises a 1750kg car 1.8m in an...Ch. 3 - Prob. 3.24PCh. 3 - A hydraulic cylinder of area 0.01m2 must push a...Ch. 3 - A hydraulic cylinder has a piston cross-sectional...Ch. 3 - A bulldozer pushes 800kg of din l00m with a force...Ch. 3 - Two hydraulic cylinders maintain a pressure of...Ch. 3 - A motor delivers 50hp on a drive shaft at 1800rpm...Ch. 3 - Solve Problem 3.24, but assume that the steam...Ch. 3 - The R4l0A in Problem 3.1 2c is at 1000kPa,50C with...Ch. 3 - A 400L tank, A (see Fig. P3.32), contains argon...Ch. 3 - A piston/cylinder assembly contains 2kg of liquid...Ch. 3 - Heat transfer to a 1.5kg block of ice at -10C...Ch. 3 - A cylinder fitted with a frictionless piston...Ch. 3 - A piston cylinder contains 2kg of water at 20C...Ch. 3 - A nitrogen gas goes through a polytropic process...Ch. 3 - Helium gas expands from 125kPa,350K and 0.25m3 to...Ch. 3 - A balloon behaves so that the pressure is P=C2V1/3...Ch. 3 - A 15cm thick concrete wall, k=1.28W/mK , has a...Ch. 3 - The brake shoe and steel drum of a car...Ch. 3 - Prob. 3.42PCh. 3 - A power plant condenser (heat exchanger) transfers...Ch. 3 - Prob. 3.44PCh. 3 - A steel Pot, with conductivity of 15W/m and a 50mm...Ch. 3 - A wall surface on a house is 30C with an...Ch. 3 - A radiant heat lamp is a rod, tong and in diameter...Ch. 3 - A radiant beating lamp has a surface temperature...Ch. 3 - Determine the phase of the following substances...Ch. 3 - Find the phase and the missing properties of P, T,...Ch. 3 - Indicate the location of the four states in...Ch. 3 - Find the missing property of P, T, y, u, h, and x...Ch. 3 - Find the missing properties for carbon dioxide at...Ch. 3 - Find the missing property of P, T, y, u, h, and x...Ch. 3 - Saturated liquid water at 20C is compressed to a...Ch. 3 - Consider a steel bottle as a CV. It contains...Ch. 3 - A piston cylinder contains water with quality 75...Ch. 3 - Problem 3.137 and write the left-hand side...Ch. 3 - Saturated vapor R410A at 0C in a rigid tank is...Ch. 3 - A constant-pressure piston/cylinder assembly...Ch. 3 - A container is split in two equal volumes by a...Ch. 3 - A cylinder fined with a frictionless piston...Ch. 3 - A piston/cylinder contains 1.5kg of water at...Ch. 3 - Ammonia (0.5kg) in a piston cy1tnde at 200kPa,10C...Ch. 3 - A water-filled reactor with a volume of 1m3 is at...Ch. 3 - A rigid 1kg steel tank holds 0.75kg ammonia at 70C...Ch. 3 - Prob. 3.68PCh. 3 - A piston/cylinder arrangement with a linear spring...Ch. 3 - Prob. 3.70PCh. 3 - Assume the same setup as in Problem 3.66. but the...Ch. 3 - A rigid steel tank contains 0.5kgR410A at 0°C with...Ch. 3 - Redo the previous problem when you also consider...Ch. 3 - Prob. 3.74PCh. 3 - Supetheated refrigerant R-134a at 20°C and 100 kPa...Ch. 3 - In a sink, 5 L of water at 70°C is combined with 1...Ch. 3 - Prob. 3.77PCh. 3 - A copper block of volume 1 L s heat treated at...Ch. 3 - A car with mass 1275 kg is driven at 60 km h when...Ch. 3 - A piston cylinder (0.5 kg steel altogether)...Ch. 3 - An engine, shown in Fig P3.81, consists of a 100kg...Ch. 3 - Use the ideal gas air A.7 to evaluate the specific...Ch. 3 - Estimate the constant specific heats for R-134a...Ch. 3 - Find the change in u for carbon dioxide between...Ch. 3 - Nitrogen at 300 K. 3 MPa is heated to 500 K Find...Ch. 3 - Repeat Problem 3.84 for nitrogen gas.Ch. 3 - Find the change in enthalpy for carbon dioxide...Ch. 3 - Water at 20°C and 100 kPa is brought to l00 kPa...Ch. 3 - Prob. 3.89PCh. 3 - A rigid container has 2 kg of oxygen gas at l00...Ch. 3 - Air (3kg) is in a piston cylinder similar to...Ch. 3 - A 10-m-high cylinder. with a cross-sectional area...Ch. 3 - A cylinder with a piston restrained by a linear...Ch. 3 - A constant pressure container is filled with 1 kg...Ch. 3 - A spring-loaded piston cylinder contains 1.5kg of...Ch. 3 - An insulated cylinder is divided into two pans of...Ch. 3 - Helium gas expands from 125 kPa, 350 K and 0.25m3...Ch. 3 - A piston cylinder device contains 0.1 kg of air at...Ch. 3 - A gasoline engine has a piston/cylinder with 0.1...Ch. 3 - Solve the previous problem using Table A.7.Ch. 3 - A piston/cylinder has nitrogen gas at 750 K and...Ch. 3 - A piston/cylinder assembly has 1 kg of propane gas...Ch. 3 - A piston cylinder arrangement of initial volume...Ch. 3 - A piston/cylinder assembly in a car contains 0.2 L...Ch. 3 - Air goes through a polytropic process with n=1.3...Ch. 3 - Saturated vapor R410A at 10°C of mass 0.6 kg is in...Ch. 3 - A helium gas heated at constant volume from 100...Ch. 3 - A piston/cylinder shown in Fig. P3.108 contains...Ch. 3 - A piston/cylinder has water at 200 kPa, x=0.5 and...Ch. 3 - Ten kilograms of water in a piston/cylinder...Ch. 3 - Water in piston/cylinder (Fig. P3.111) is a...Ch. 3 - A setup lake the one in Fig P3.108 has the R-410A...Ch. 3 - The piston/cylinder inFig. P3.113 contains 0.1 kg...Ch. 3 - A piston/cylinder arrangement contains 5 kg of...Ch. 3 - A piston/cylinder setup similar to Problem 3.110...Ch. 3 - A piston/cylinder contains air at 1000 kPa, 800 K...Ch. 3 - Prob. 3.117PCh. 3 - A 100-hp car engine has a drive shaft rotating at...Ch. 3 - Prob. 3.119PCh. 3 - As fresh-poured concrete hardens, the chemical...Ch. 3 - A 1.2-kg pot of water at 20°C is put on a stove...Ch. 3 - A computer in a closed room of volume 200m3...Ch. 3 - A 500-W heater is used to melt 2 kg of solid ice...Ch. 3 - A 3-kg mass of nitrogen gas at 2000 K, V=C , cools...Ch. 3 - Electric power as volts times amperes (P=Vi) ....Ch. 3 - A copper wire of diameter 2 mm is 10m long and...Ch. 3 - A battery is well insulated while being charged by...Ch. 3 - A sheet of rubberis stretched out over a ring of...Ch. 3 - Assume a balloon material with a constant surface...Ch. 3 - A soap bubble has a surface tension of =3104N/cm...Ch. 3 - According to Table 3.4 residential buildings in US...Ch. 3 - total energy use in the US from Table 3.4 for 2011...Ch. 3 - A wind turbine with 20m diameter rotors spins at...Ch. 3 - Prob. 3.134PCh. 3 - A house is being designed to use a thick concrete...Ch. 3 - A solar pond with 20°C salt water, Cp=3.8kJ/kg-K...Ch. 3 - Prob. 3.137PCh. 3 - A rigidtank is divided into tworooms,both...Ch. 3 - A piston/cylinder has a water volume separated in...Ch. 3 - The cylinder volume below the constant loaded...Ch. 3 - Air in tank B is at 200 kPa, 280 K and mass 1 kg....Ch. 3 - A piston/cylinder setup (Fig P3.110) contains 1 kg...Ch. 3 - Two kilograms of water is contained in a...Ch. 3 - A piston cylinder has 0.1 kg water at x=0.5 ,...Ch. 3 - A piston/cylinder arrangement has the piston...Ch. 3 - A vertical/cylinder (Fig. P3.146) has a 61.18-kg...Ch. 3 - Water in a piston/cylinder, similar to Fig P3.110,...Ch. 3 - A rigid container has two rooms filled with water,...Ch. 3 - Prob. 3.149PCh. 3 - A piston/cylinder setup, similar to Fig. P3.143,...Ch. 3 - A spherical balloon contains 2 kg of R-410A at 0°C...Ch. 3 - Prob. 3.152PCh. 3 - Prob. 3.153EPCh. 3 - Work as Fx has units of lbf ft. What is that in...Ch. 3 - Work in the expression in Eq. 3.18 or Eq. 3.22...Ch. 3 - Prob. 3.156EPCh. 3 - Prob. 3.157EPCh. 3 - You heat a gas 20 R at P=C . Which gas in Table...Ch. 3 - A piston motion moves a 50-lbm hammerhead...Ch. 3 - A pump pushes 35000ft3 of liquid water at 60 F up...Ch. 3 - Prob. 3.161EPCh. 3 - Prob. 3.162EPCh. 3 - Prob. 3.163EPCh. 3 - A car with tires of outer radius 12 in. drives...Ch. 3 - The R-410A in Problem 3.9(c) is at 150 psia, 120 F...Ch. 3 - Prob. 3.166EPCh. 3 - A nitrogen gas goes through apolytropic process...Ch. 3 - Find the rate of conduction heat transfer per unit...Ch. 3 - The sun shines on a 1500-ft2 road surface so that...Ch. 3 - Find the missing properties and give the phase of...Ch. 3 - Find the missing properties among (P,T,v,u,h)...Ch. 3 - Find the missing properties among (P,T,v,u,h)...Ch. 3 - Saturated vapor R-410A at 60 F in a rigid tank is...Ch. 3 - A containeris split in two equal volumes by a...Ch. 3 - Saturated vapor R-410A at 200 psia in a...Ch. 3 - A cylinder fitted with a frictionless piston...Ch. 3 - Prob. 3.177EPCh. 3 - A water-filled reactor with a volume of 50ft3 is...Ch. 3 - Prob. 3.179EPCh. 3 - A piston/cylinder arrangement with a linear spring...Ch. 3 - Prob. 3.181EPCh. 3 - Prob. 3.182EPCh. 3 - Prob. 3.183EPCh. 3 - Prob. 3.184EPCh. 3 - Prob. 3.185EPCh. 3 - A closed rigid container is filled with 3 lbm...Ch. 3 - An insulated cylinder is divided into two parts of...Ch. 3 - Helium gas expands from 20 psia, 600 R, and 9ft3...Ch. 3 - Prob. 3.189EPCh. 3 - A cylinder fitted with a frictionless piston...Ch. 3 - Prob. 3.191EPCh. 3 - A piston/cylinder contains air at 150 psia, 1400 R...Ch. 3 - A piston/cylinder has 2 lbm of R-134a at state 1...Ch. 3 - A force of 300 lbf moves a truck at a speed of 40...Ch. 3 - Prob. 3.195EPCh. 3 - Water is in a piston/cylinder maintaining constant...Ch. 3 - A mass of 6 lbm nitrogen gas at 3600 R, V=C ,...Ch. 3 - Prob. 3.198EPCh. 3 - Ammonia is contained in a sealed, rigid tank at 30...Ch. 3 - Water in a piston/cylinder, similar to Fig....
Additional Engineering Textbook Solutions
Find more solutions based on key concepts
The ____________ is always transparent.
Web Development and Design Foundations with HTML5 (8th Edition)
a. Suppose you XOR the first 2 bits of a string of bits and then continue down the string by successively XORin...
Computer Science: An Overview (13th Edition) (What's New in Computer Science)
What is an algorithm?
Starting Out With Visual Basic (8th Edition)
41. An ideal gas, kept in a 5-liter [L] container at 300 kelvins [K], exhibits a pressure of 2 atmospheres [atm...
Thinking Like an Engineer: An Active Learning Approach (4th Edition)
17–1C A high-speed aircraft is cruising in still air. How does the temperature of air at the nose of the aircra...
Thermodynamics: An Engineering Approach
Show a snippet of PHP code for displaying the contents of a recordset. Explain the meaning of the code.
Database Concepts (8th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Similar questions
- A single-pass crossflow heat exchanger is used to cool jacket water (cp = 1.0 Btu/lbm.°F) of a diesel engine from 190°F to 140°F, using air (Cp = 0.245 Btu/lbm.°F) at inlet temperature of 90°F. Both air flow and water flow are unmixed. If the water and air mass flow rates are 85500 lbm/h and 400,000 lbm/h, respectively, determine the log mean temperature difference for this heat exchanger. Assume the correction factor F to be 0.92. Air flow (unmixed) Water flow (unmixed) The log mean temperature difference of the heat exchanger is °F.arrow_forwardusing the theorem of three moments, find all the reactions and supports, I need concise calculations only. the answers are at the bottom, I need concise steps and minimal explanationsarrow_forwardIn an industrial facility, a counter-flow double-pipe heat exchanger uses superheated steam at a temperature of 155°C to heat feed water at 30°C. The superheated steam experiences a temperature drop of 70°C as it exits the heat exchanger. The water to be heated flows through the heat exchanger tube of negligible thickness at a constant rate of 3.47 kg/s. The convective heat transfer coefficient on the superheated steam and water side is 850 W/m²K and 1250 W/m²K, respectively. To account for the fouling due to chemical impurities that might be present in the feed water, assume a fouling factor of 0.00015 m² K/W for the water side. The specific heat of water is determined at an average temperature of (30+70)°C/2 = 50°C and is taken to be Cp J/kg-K. Water Steam Determine the heat exchanger area required to maintain the exit temperature of the water to a minimum of 70°C. The heat exchanger area required isarrow_forward
- Stress, ksi 160 72 150- 140 80 70 ༄ ྃ ༈ ཎྜ རྦ ༅ ཎྜ ྣཧྨ ➢ 130 120 110 100 90 2.0 2.8 3.6 4.4 5 Wire diameter, mm 6.0 6.8 2 7.6 8.4 Compression and extension springs. ASTM A227 Class II Light service Average service 0.020 0.060 0.100 0.140 0.180 0.220 0.260 0.300 0.340 0.380 0.420 0.460 0.500 Wire diameter, in Torsional stress due to initial tension, ksi 10 ४ 20 Preferred range 100 Stress, MPa 9.2 10.0 10.8 11.6 12.4 1100 1035 965 895 825 760 Severe service 690 620 550 50 150 3456789 10 11 12 13 14 15 16 Spring index, C = DJD FIGURE 18-21 Recommended torsional shear stress in an extension spring due to initial tension (Data from Associated Spring, Barnes Group, Inc.) 50 200 485 Stress, MPaarrow_forwardBolted Joint Design Bolted Frames Total Force due to door weight: P = 240 lb Number of Bolts: N = Distance to Bolt C/L: a = 4 N/A Bolt Material - Allowable shear stress of bolt material: T₂ = x Distance from Bolt centroid to bolt: x = y Distance from Bolt centroid to bolt: y = Degrees per Radian- Results y-Load on each bolt: F, = Moment resisted by bolt pattern: M = Radial distance from Bolt centroid to bolt: r = Sum squares of all radial distances: Σr² Force on each bolt to resist moment: F, - Angle for force composition: e= X-Force on each bolt to resist moment: F- y-Force on each bolt to resist moment: Fly Total y-Force on each bolt: Fy = Resultant force on bolt 1: R₁ = Required shear stress area for a bolt: A₂ = ASTM Grade A307 Steel 10,000 0 psi from Table 20-1 3.0 57.296 in degrees lb per bolt lb-in Formula FS-P/N M-Px XB r = (x² + y²)0.5 in² Σ 4r² Mr F₁ = Στ lb degrees lb lb lb Minimum Bolt Diameter: Din = Rounded up Bolt Diameter: D = 55 P. 1.5 in 2 in (3x) 1 in This bracket…arrow_forwardUniversity of Babylon Collage of Engineering/ Al-Musayab Department of Automobiles Final Examination/ Stage: 3rd Notes: Answer 4 questions only 2023-2202 Subject: Theory of vehicles Date: 2023\06\10-Saturday Time: Three Hours Course 2nd Attempt 1st Q1: A Hooke's coupling connects two shafts whose axes are inclined at 30°. The of the driven shaft? Find the maximum value of retardation or acceleration and driving shaft rotates uniformly at 600 rpm. What are the extreme angular velocities state the angle where both will occur. (12.5 Marks) Q2: Four masses, A, B, C, and D), revolve at equal radii and are equally spaced along a shaft. The mass B is 7 kg, and the radius of C and D make angles of 90° and 240°, respectively, with the radius of B. Find the magnitude of the masses A, C, and D and the angular position of A so that the system may be completely balanced. (12.5 Marks) Q3: A cam has straight worked faces that are tangential to a base circle of diameter 90 mm. The follower is a roller…arrow_forward
- Problem 18-26 Added Extension Springs Spring Material ASTM A227 Modulus of Elasticity of the Material in Shear: G 1.150E+07 psi Average Service Max Operating Load: F₁ = 100 lb Max Length between attachment points: L₁ = 60.00 in 20.00 lb 26.00 1.400 Min Operating Load: F₁ = Min Length between attachment points: L₁ = Maximum Outside Diameter = in in Results Note: you select a wire diameter from the "US steel wire gage" column in table 18-2 Formula k = AF/AL k = (F0-F1)/(Lo - L₁) Spring Rate: k = lb/in Assumed Trial Outside Diameter: OD = Assumed Trial Mean: D ma Assumed Design Stress in Spring: Tda in 1.070 in 102,000 psi Assumed Wahl Factor: K = 1.2 Calculated Wire Diameter: Dwa Actual Wire Diameter: Dw Actual outer diameter: OD = Actual inner diameter: ID= Spring Index: C = See Figure 18-8 Dw= [8KF Dm πTd 1/3 in 5' 5' 5' 5' This corresponds to US Steel 9 wire gage ID = Dm - Dw C = Dm/Dw 4C - 1 0.615 K = + 4C - с Wahl Factor: K = 8KFDm 8KFC T = TD πD Stress in Spring at F = Fo: To psi…arrow_forwardCHAIN DRIVE DESIGN Initial Input Data: Application: Garage Door Opener Drive type: AC Motor Driven machine Chain and Sprocket to pull the door up Degrees per Radian: 57.2958 degrees Sprocket Diameter: D = 1.690 in Number of strands: Chain number: 1 40 Service factor: 1.3 Table 7-10 No. of teeth Computed Data: Actual Motor Power Input: 0.000 hp Sprocket Speed (for sprocket attached to gear shaft) Design power: 0.00 rpm 0 hp 11 12 0.06 0.15 0.29 0.56 0.99 1.09 1.61 2.64 TABLE 7-7 Horsepower Ratings-Single Strand Roller Chain No. 40 0.500 inch pitch 10 25 50 100 180 200 300 500 700 900 1000 1: 0.06 0.14 0.27 0.52 0.91 1.00 1.48 2.42 3.34 4.25 4.70 ! 3.64 4.64 5.13 13 0.07 0.16 0.31 0.61 1.07 1.19 1.75 2.86 3.95 5.02 5.56 Design Decisions-Chain Type and Teeth Numbers: 14 Chain number: Use Table 7-7 Chain pitch: p = in 15 Number of Teeth: N = Per Table 7-7 16 0.08 0.20 0.39 0.75 1.32 1.46 2.15 3.52 0.07 0.17 0.34 0.66 1.15 1.28 1.88 3.08 0.08 0.19 0.36 0.70 1.24 1.37 2.02 3.30 4.55 5.80…arrow_forwardInput Data: Torque needed to overcome rolling friction in rollers, slides and other moving parts, except for Motor and Worm Gear the worm gear T₁ = Length of travel of door: Time for door to open or close: LD = 50 lb-in. 90 in t= 12.5 seconds Pitch diameter for chain sprocket: DPC 1.690 in Weight of Door: P = No. of worm threads: Nw = Worm Pitch diameter: Dw Diametral pitch: Pd Normal pressure angle: Degrees per Radian: Number of gear teeth: Calculated Data: Linear velocity of door and chain (in/sec): Linear velocity of door and chain (ft/min): Output Speed of Gear and Sprocket: Upward Force due to Weight of Door: Фо = = NG= 240 lb 2 1.250 in 12 14.5 degrees 57.2958 degrees 28 Vα= in/sec VC= ft/min NG = rpm FD lb Net Upward Force on Door: Fou lb Torque on gear ignoring rolling friction: TG = lb-in. Formula = FDU FD-2 x Fo (note: Fo is the Max Operating load of the extension springs). This is also the initial tension in the chain. TG = FDU X DPC/2 This is the also the torque on the…arrow_forward
- Q5/A: A car with a track of 1.5 m and a wheelbase of 2.9 m has a steering gear mechanism of the Ackermann type. The distance between the front stub axle pivots is 1.3 m. The length of each track arm is 150 mm, and the length of the track rod is 1.2 m. Find the angle turned through by the outer wheel if the angle turned through by the inner wheel is 30°. (6 Marks) Q5/B: Write True on the correct sentences and False on the wrong sentences listed below:- 1- In automobiles, the power is transmitted from the gearbox to the differential through bevel gears. 2- The minimum radius circle drawn to the cam profile is called the base circle. 3- The Proell governor, compared to the Porter governor, has less lift at the same speed. 4- The balancing of rotating and reciprocating parts of an engine is necessary when it runs at a slow speed. (6.5 Marks) ***Best of Luck *** جامعة بابل UNIVERSITY OF BABYLON Examiner: Mohanad R. Hameed Head of Department: Dr. Dhyai H. Jawadarrow_forwardUniversity of Babylon Collage of Engineering/ Al-Musayab Department of Automobiles Mid Examination/ Stage: 3rd Subject: Theory of Vehicles Date: 14 \ 4 \2025 Time: 1.5 Hours 2025-2024 Q1: The arms of a Porter governor are 250 mm long. The upper arms are pivoted on the axis of revolution, but the lower arms are attached to a sleeve at a distance of 50 mm from the axis of rotation. The weight on the sleeve is 600 N and the weight of each ball is 80 N. Determine the equilibrium speed when the radius of rotation of the balls is 150 mm. If the friction is equivalent to a load of 25 N at the sleeve, determine the range of speed for this position. Q2: In a loaded Proell governor shown in Figure below each ball weighs 3 kg and the central sleeve weighs 25 kg. The arms are of 200 mm length and pivoted about axis displaced from the central axis of rotation by 38.5 mm, y=238 mm, x=303.5 mm, CE 85 mm, MD 142.5 mm. Determine the equilibrium speed. Fe mg E M 2 Q3: In a spring loaded Hartnell type…arrow_forwardusing the theorem of three moments, find all the reactions and supports, I need the calculations onlyarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY

Elements Of Electromagnetics
Mechanical Engineering
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:9780134319650
Author:Russell C. Hibbeler
Publisher:PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:9781259822674
Author:Yunus A. Cengel Dr., Michael A. Boles
Publisher:McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:9781118170519
Author:Norman S. Nise
Publisher:WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:9781337093347
Author:Barry J. Goodno, James M. Gere
Publisher:Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:9781118807330
Author:James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:WILEY
First Law of Thermodynamics, Basic Introduction - Internal Energy, Heat and Work - Chemistry; Author: The Organic Chemistry Tutor;https://www.youtube.com/watch?v=NyOYW07-L5g;License: Standard youtube license