
Concept explainers
(a)
Interpretation:
Using VSEPR Theory, the geometry of each central atom present in given structure of Androstenedione is to be identified.
Concept Introduction:
Bond angles in the molecules can be predicted by using valence shell electron pair repulsion (VSEPR) model. According to this model, the valence electrons of an atom are involved in the formation of single, double or triple bond. The valence electrons can also be unshared and exist as lone pair on atoms. The combination forms a negatively charged region of electron density around a nucleus. Since, like charges do not attract, the region of electron density around a nucleus spread out so that each atom is as far away from each other at different angles.
(b)
Interpretation:
Using VSEPR theory, the various relative bond angles associated with each central atom of the Androstenedione molecule is to be determined.
Concept Introduction:
Bond angles in the molecules can be predicted by using valence shell electron pair repulsion (VSEPR) model. According to this model, the valence electrons of an atom are involved in the formation of single, double or triple bond. The valence electrons can also be unshared and exist as lone pair on atoms. The combination forms a negatively charged region of electron density around a nucleus. Since, like charges do not attract, the region of electron density around a nucleus spread out so that each atom is as far away from each other at different angles.
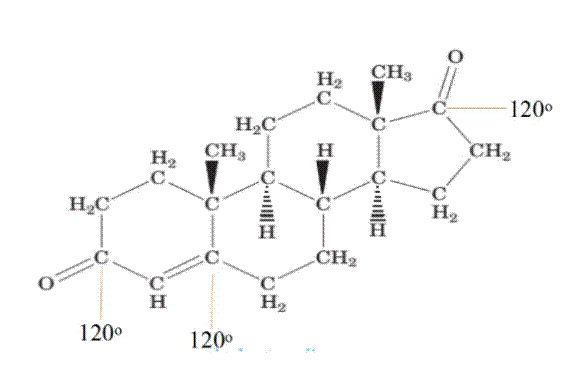
1. Each carbon atom having all single bonds having tetrahedral geometry has bond angle
2. Each carbon atom having double bond having trigonal planner geometry has bond angle 120(.
(c)
Interpretation:
The most polar bond in androstenedione is to be predicted.
Concept Introduction:
A molecule is polar if it has polar bonds and the centers of its partial positive and partial negative charge do not coincide.
(d)
Interpretation:
Observing the bonds of Androstenedione molecule, it should be determined whether Androstenedione is polar or nonpolar.
Concept Introduction:
A molecule is polar if it has polar bonds and if the centers of partial positive charge and partial negative charge lie at different places with in the molecule.
Want to see the full answer?
Check out a sample textbook solution
Chapter 3 Solutions
Bundle: Introduction to General, Organic and Biochemistry, 11th + OWLv2, 4 terms (24 months) Printed Access Card
- Predict the major organic product(s) for the following reactions.arrow_forwardProvide the complete mechanism for the reactions below. You must include appropriate arrows,intermediates, and formal charges.arrow_forwardIndicate the products obtained by reacting fluorobenzene with a sulfonitric mixture.arrow_forward
- If I have 1-bromopropene, to obtain compound A, I have to add NaOH and another compound. Indicate which compound that would be. C6H5 CH3arrow_forwardIf I have 1-bromopropene and I want to obtain (1,1-dipropoxyethyl)benzene, indicate the compound that I should add in addition to NaOH.arrow_forwardDraw the major product of this reaction. Ignore inorganic byproducts. Ο HSCH2CH2CH2SH, BF3 Select to Draw I Submitarrow_forward
- Feedback (7/10) Draw the major product of this reaction. Ignore inorganic byproducts. Assume that the water side product is continuously removed to drive the reaction toward products. Incorrect, 3 attempts remaining Ο (CH3CH2)2NH, TSOH Select to Draw V N. 87% Retryarrow_forwardIf I want to obtain (1,1-dipropoxyethyl)benzene from 1-bromopropene, indicate the product that I have to add in addition to NaOH.arrow_forwardIndicate the products obtained when fluorobenzene reacts with a sulfonitric acid mixture (HNO3 + H2SO4). Indicate the majority if necessary.arrow_forward
- Indicate the products obtained when chlorobenzene acid reacts with a sulfonitric acid mixture (HNO3 + H2SO4). Indicate the majority if necessary.arrow_forwardIndicate the products obtained by reacting benzenesulfonic acid with a sulfonitric acid mixture (HNO3 + H2SO4). Indicate the majority if necessary.arrow_forwardIndicate the products obtained by reacting ethylbenzene with a sulfonitric acid mixture (HNO3 + H2SO4). Indicate the majority if necessary.arrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning

