
3-23 Predict which ions are stable:
(a) 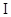
(b) 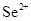
(c) 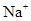
(d) 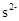
(e) 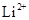
(f) 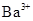
Interpretation:
Whether
Concept Introduction:
Octet rule: generally all atoms will lose, gain or share electrons to attain 8 valence electrons; the electronic configuration of the nearest noble element.
Duet rule: generally some atoms like hydrogen, lithium, beryllium will lose, gain or share electrons to attain 2 valence electrons; the electronic configuration of the nearest noble element like helium.
Answer to Problem 3.23P
Explanation of Solution
The atomic number or number of electrons of
When
The electronic configuration of the nearest noble element like helium, neon argon is the stable configuration hence the
Interpretation:
Whether
Concept Introduction:
Octet rule: generally all atoms will lose, gain or share electrons to attain 8 valence electrons; the electronic configuration of the nearest noble element.
Duet rule: generally some atoms like hydrogen, lithium, beryllium will lose, gain or share electrons to attain 2 valence electrons; the electronic configuration of the nearest noble element like helium.
Answer to Problem 3.23P
Explanation of Solution
The atomic number or number of electrons of
When
The electronic configuration of the nearest noble element like helium, neon argon is the stable configuration hence the
Interpretation:
Whether
Concept Introduction:
Octet rule: generally all atoms will lose, gain or share electrons to attain 8 valence electrons; the electronic configuration of the nearest noble element.
Duet rule: generally some atoms like hydrogen, lithium, beryllium will lose, gain or share electrons to attain 2 valence electrons; the electronic configuration of the nearest noble element like helium.
Answer to Problem 3.23P
Explanation of Solution
The atomic number or number of electrons of
When
The electronic configuration of the nearest noble element like helium, neon argon is the stable configuration hence the
Interpretation:
Whether
Concept Introduction:
Octet rule: generally all atoms will lose, gain or share electrons to attain 8 valence electrons; the electronic configuration of the nearest noble element.
Duet rule: generally some atoms like hydrogen, lithium, beryllium will lose, gain or share electrons to attain 2 valence electrons; the electronic configuration of the nearest noble element like helium.
Answer to Problem 3.23P
Explanation of Solution
The atomic number or number of electrons of
When
The electronic configuration of the nearest noble element like helium, neon argon is the stable configuration hence the
Interpretation:
Whether
Concept Introduction:
Octet rule: generally all atoms will lose, gain or share electrons to attain 8 valence electrons; the electronic configuration of the nearest noble element.
Duet rule: generally some atoms like hydrogen, lithium, beryllium will lose, gain or share electrons to attain 2 valence electrons; the electronic configuration of the nearest noble element like helium.
Answer to Problem 3.23P
Explanation of Solution
The atomic number or number of electrons of
When
The electronic configuration of the nearest noble element like helium, neon argon is the stable configuration hence the
Interpretation:
Whether
Concept Introduction:
Octet rule: generally all atoms will lose, gain or share electrons to attain 8 valence electrons; the electronic configuration of the nearest noble element.
Duet rule: generally some atoms like hydrogen, lithium, beryllium will lose, gain or share electrons to attain 2 valence electrons; the electronic configuration of the nearest noble element like helium.
Answer to Problem 3.23P
Explanation of Solution
The atomic number or number of electrons of
When
The electronic configuration of the nearest noble element like helium, neon argon is the stable configuration hence the
Want to see more full solutions like this?
Chapter 3 Solutions
Bundle: Introduction to General, Organic and Biochemistry, 11th + OWLv2, 4 terms (24 months) Printed Access Card
- Identify any polar covalent bonds in epichlorohydrin with S+ and 8- symbols in the appropriate locations. Choose the correct answer below. Η H's+ 6Η Η Η Η Η Ηδ Η Ο Ο HH +Η Η +Η Η Η -8+ CIarrow_forwardH H:O::::H H H HH H::O:D:D:H HH HH H:O:D:D:H .. HH H:O:D:D:H H H Select the correct Lewis dot structure for the following compound: CH3CH2OHarrow_forwardRank the following compounds in order of decreasing boiling point. ннннн -С-С-Н . н-с- ННННН H ΗΤΗ НННН TTTĪ н-с-с-с-с-о-н НННН НН C' Н н-с-с-с-с-н НН || Ш НННН H-C-C-C-C-N-H ННННН IVarrow_forward
- Rank the following compounds in order of decreasing dipole moment. |>||>||| ||>|||>| |>|||>|| |||>||>| O ||>>||| H F H F H c=c || H c=c F F IIIarrow_forwardchoose the description that best describes the geometry for the following charged species ch3-arrow_forwardWhy isn't the ketone in this compound converted to an acetal or hemiacetal by the alcohol and acid?arrow_forward
- What is the approximate bond angle around the nitrogen atom? HNH H Harrow_forwardOH 1. NaOCH2CH3 Q 2. CH3CH2Br (1 equiv) H3O+ Select to Draw 1. NaOCH2 CH3 2. CH3Br (1 equiv) heat Select to Edit Select to Drawarrow_forwardComplete and balance the following half-reaction in acidic solution. Be sure to include the proper phases for all species within the reaction. S₂O₃²⁻(aq) → S₄O₆²⁻(aq)arrow_forward
- Q Select to Edit NH3 (CH3)2CHCI (1 equiv) AICI 3 Select to Draw cat. H2SO4 SO3 (1 equiv) HO SOCl2 pyridine Select to Edit >arrow_forwardComplete and balance the following half-reaction in basic solution. Be sure to include the proper phases for all species within the reaction. Zn(s) → Zn(OH)₄²⁻(aq)arrow_forwardb. ὋΗ CH3CH2OH H2SO4arrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
