
(a)
Interpretation:
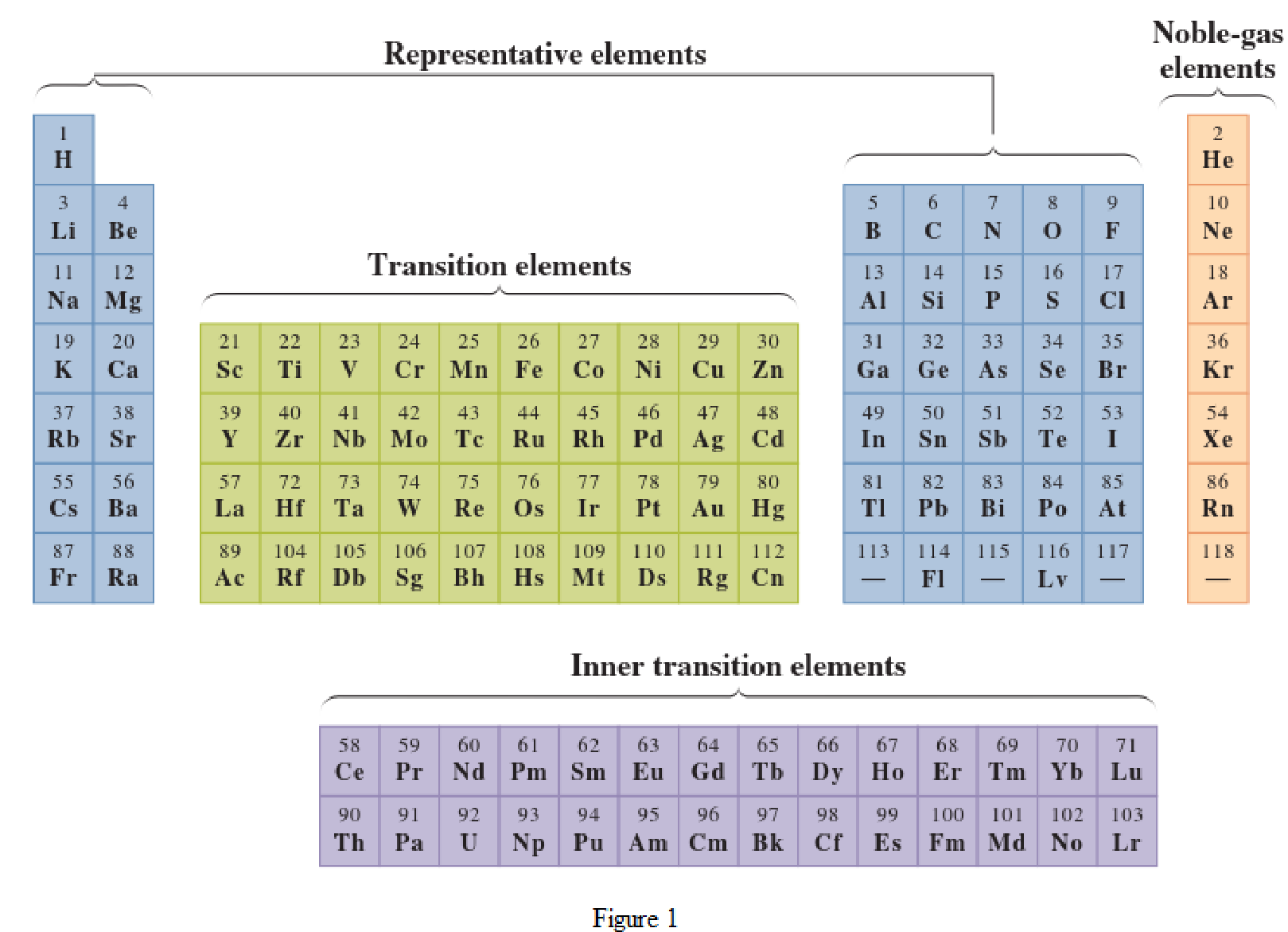
In the given periodic table, how many elements those are highlighted which represent inner
Concept Introduction:
Elements in the periodic table are classified in several different ways and out of them two most common systems are,
- System based on the physical properties in which they are classified as metals and nonmetals.
- System based on electronic configuration in which they are classified as noble-gas, representative elements, transition elements, or inner-transition elements.
Noble-gas elements are the ones that are located in far right of periodic table. The physical state of these elements at room temperature is gas. The noble gases have their electronic configuration ending with
Representative elements are the ones that are in s area and area of the periodic table. They have partially filled s subshell or p subshell in their electronic configurations. Some of the elements are nonmetals while others are metals.
Transition elements are the ones that are located in d area of periodic table. They have the distinguishing electrons in their d subshell. All the transition elements are metals.
Inner transition elements are the ones that are located in f area of the periodic table. They have the distinguishing electrons in their f subshell. All inner transition elements are metals.
(b)
Interpretation:
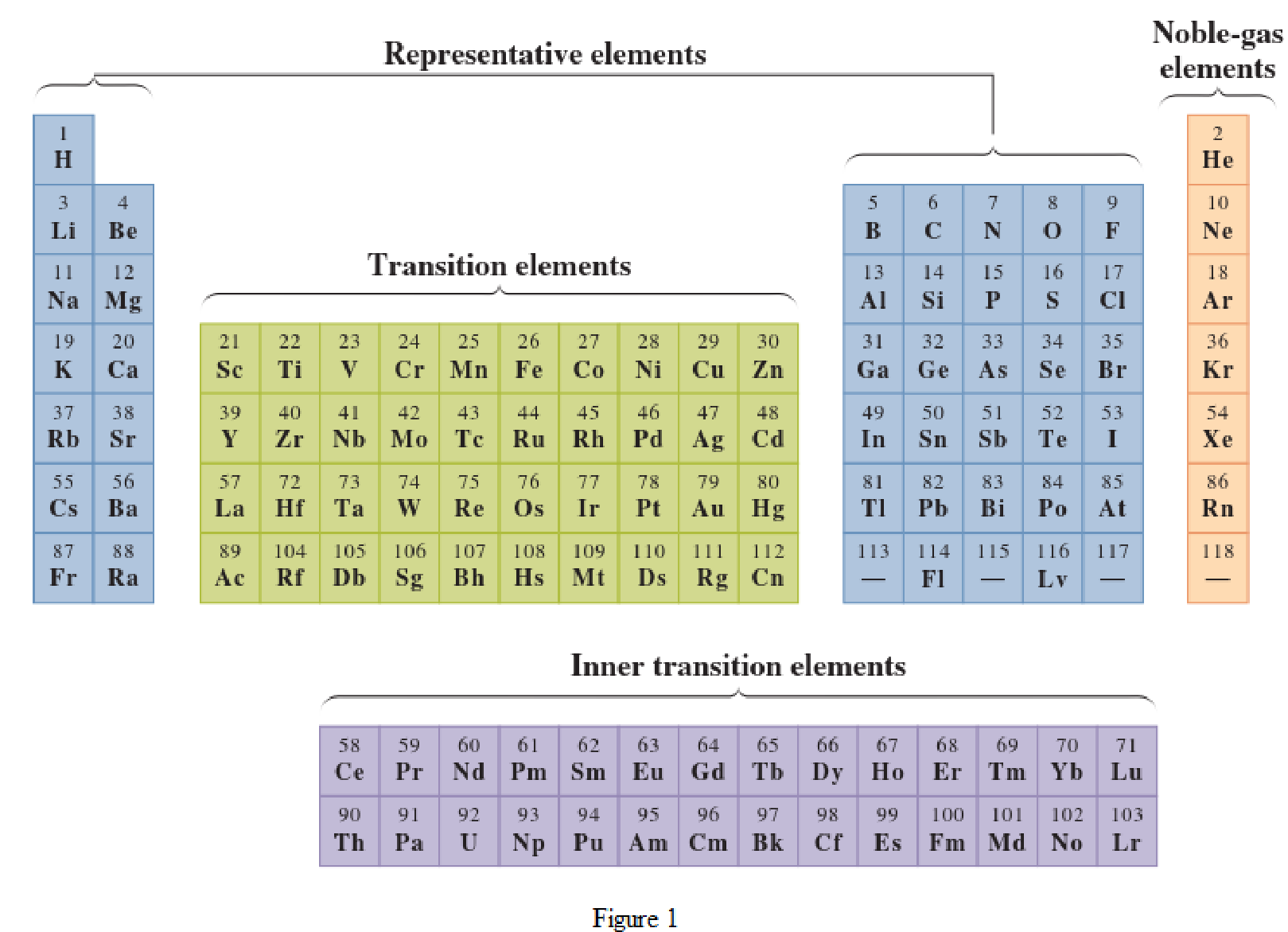
In the given periodic table, how many elements those are highlighted which represent transition have to be determined.
Concept Introduction:
Elements in the periodic table are classified in several different ways and out of them two most common systems are,
- System based on the physical properties in which they are classified as metals and nonmetals.
- System based on electronic configuration in which they are classified as noble-gas, representative elements, transition elements, or inner-transition elements.
Noble-gas elements are the ones that are located in far right of periodic table. The physical state of these elements at room temperature is gas. The noble gases have their electronic configuration ending with
Representative elements are the ones that are in s area and area of the periodic table. They have partially filled s subshell or p subshell in their electronic configurations. Some of the elements are nonmetals while others are metals.
Transition elements are the ones that are located in d area of periodic table. They have the distinguishing electrons in their d subshell. All the transition elements are metals.
Inner transition elements are the ones that are located in f area of the periodic table. They have the distinguishing electrons in their f subshell. All inner transition elements are metals.
(c)
Interpretation:
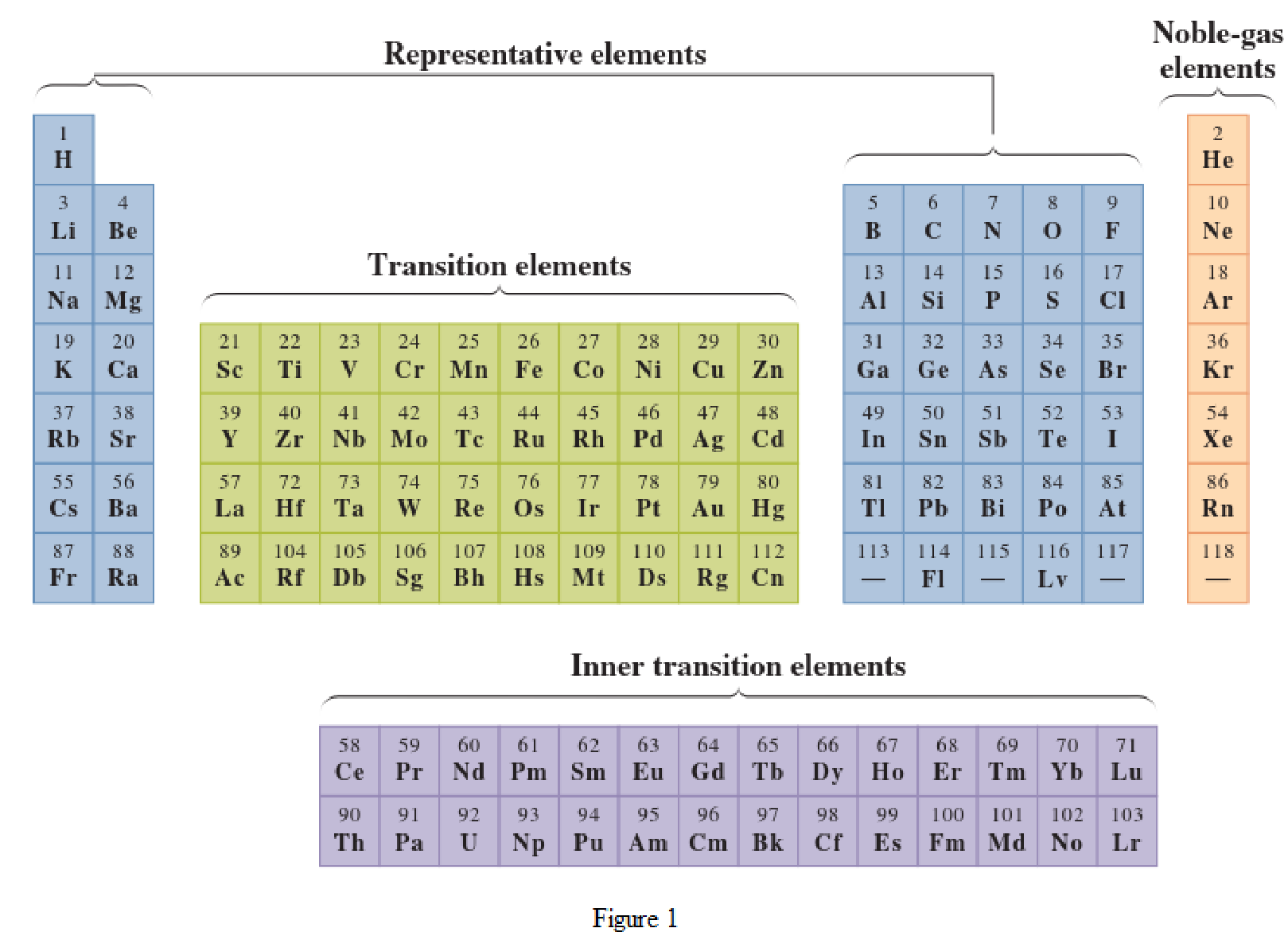
In the given periodic table, how many elements those are highlighted which represent metallic representative elements have to be determined.
Concept Introduction:
Elements in the periodic table are classified in several different ways and out of them two most common systems are,
- System based on the physical properties in which they are classified as metals and nonmetals.
- System based on electronic configuration in which they are classified as noble-gas, representative elements, transition elements, or inner-transition elements.
Noble-gas elements are the ones that are located in far right of periodic table. The physical state of these elements at room temperature is gas. The noble gases have their electronic configuration ending with
Representative elements are the ones that are in s area and area of the periodic table. They have partially filled s subshell or p subshell in their electronic configurations. Some of the elements are nonmetals while others are metals.
Transition elements are the ones that are located in d area of periodic table. They have the distinguishing electrons in their d subshell. All the transition elements are metals.
Inner transition elements are the ones that are located in f area of the periodic table. They have the distinguishing electrons in their f subshell. All inner transition elements are metals.
(d)
Interpretation:
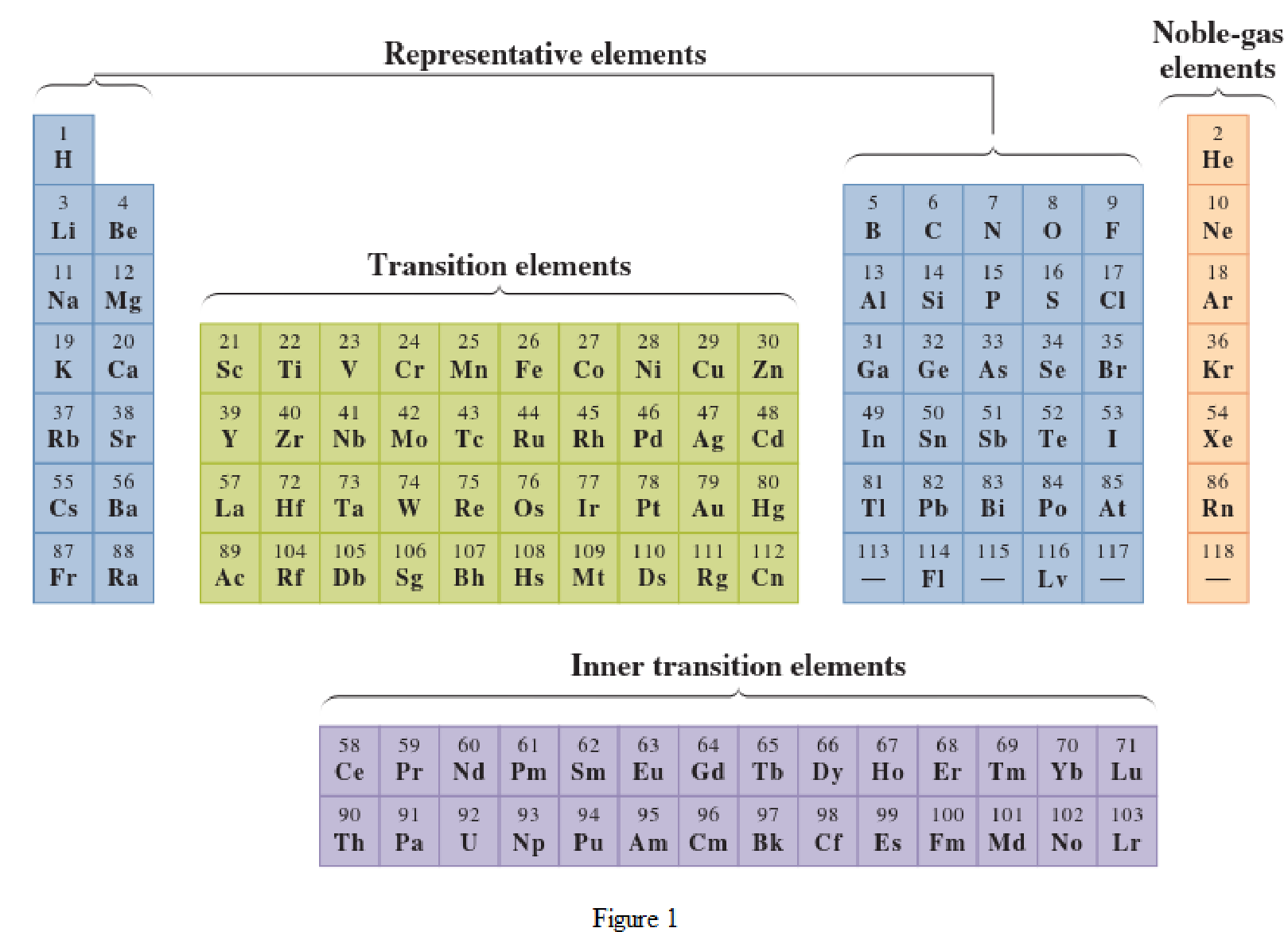
In the given periodic table, how many elements those are highlighted which represent nonmetals have to be determined.
Concept Introduction:
Elements in the periodic table are classified in several different ways and out of them two most common systems are,
- System based on the physical properties in which they are classified as metals and nonmetals.
- System based on electronic configuration in which they are classified as noble-gas, representative elements, transition elements, or inner-transition elements.
Noble-gas elements are the ones that are located in far right of periodic table. The physical state of these elements at room temperature is gas. The noble gases have their electronic configuration ending with
Representative elements are the ones that are in s area and area of the periodic table. They have partially filled s subshell or p subshell in their electronic configurations. Some of the elements are nonmetals while others are metals.
Transition elements are the ones that are located in d area of periodic table. They have the distinguishing electrons in their d subshell. All the transition elements are metals.
Inner transition elements are the ones that are located in f area of the periodic table. They have the distinguishing electrons in their f subshell. All inner transition elements are metals.
Want to see the full answer?
Check out a sample textbook solution
Chapter 3 Solutions
General, Organic, and Biological Chemistry Seventh Edition
- Draw the major product of this reaction. Ignore inorganic byproducts. N S S HgCl2, H2SO4 く 8 W X Parrow_forwardtab esc く Drawing the After running various experiments, you determine that the mechanism for the following reaction occurs in a step-wise fashion. Br + OH + Using this information, draw the correct mechanism in the space below. 1 Explanation Check F2 F1 @2 Q W A os lock control option T S # 3 80 F3 Br $ 4 0105 % OH2 + Br Add/Remove step X C F5 F6 6 R E T Y 29 & 7 F D G H Click and drag to start drawing a structure. © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Ce A F7 DII F8 C Ո 8 * 9 4 F10 F C J K L C V Z X B N M H command P ge Coarrow_forwardIndicate compound A that must react with ethylbenzene to obtain 4-ethylbenzene-1-sulfonic acid. 3-bromo-4-ethylbenzene-1-sulfonic acid.arrow_forward
- Part 1 of 2 Draw the structure of A, the minor E1 product of the reaction. esc I Skip Part Check H₂O, D 2 A + Click and drag to start drawing a structure. -0- F1 F2 1 2 # 3 Q A 80 F3 W E S D F4 $ 4 % 5 F5 ㅇ F6 R T Y F G X 5 & 7 + Save 2025 McGraw Hill LLC. All Rights Reserved. DII F7 F8 H * C 80 J Z X C V B N 4 F9 6arrow_forwardFile Preview The following is a total synthesis of the pheromone of the western pine beetle. Such syntheses are interesting both because of the organic chemistry, and because of the possibility of using species specific insecticides, rather than broad band insecticides. Provide the reagents for each step. There is some chemistry from our most recent chapter in this synthesis, but other steps are review from earlier chapters. (8 points) COOEt COOEt A C COOEt COOEt COOH B OH OTS CN D E See the last homework set F for assistance on this one. H+, H₂O G OH OH The last step is just nucleophilic addition reactions, taking the ketone to an acetal, intramolecularly. But it is hard to visualize the three dimensional shape as it occurs. Frontalin, pheromone of the western pine beetlearrow_forwardFor the reaction below: 1. Draw all reasonable elimination products to the right of the arrow. 2. In the box below the reaction, redraw any product you expect to be a major product. C Major Product: Check + ◎ + X ง © Cl I F2 80 F3 I σ F4 I F5 NaOH Click and drawing F6 A 2025 McGraw Hill LLC. All Rights E F7 F8 $ # % & 2 3 4 5 6 7 8 Q W E R T Y U A S D F G H Jarrow_forward
- Can I please get help with this graph. If you can show exactly where it needs to pass through.arrow_forwardN Draw the major product of this reaction. Ignore inorganic byproducts. D 1. H₂O, pyridine 2. neutralizing work-up V P W X DE CO e C Larrow_forwardDraw the major product of this reaction. Ignore inorganic byproducts. N O' 1. H2O, pyridine 2. neutralizing work-up く 8 W aarrow_forward
- Ideal Gas Law Practice Name If you need a refresher on Ideal Gas Law, go back to your Ideal Gas Law Reading Assignment from last week! On all of the following, you'll need to make sure to convert pressures to atm and convert temperatures to Kelvin in order to be able to use the R gas constant on your equation sheet! Given: Ideal Gas Law = then P= pressure V = volume R= ideal gas consent PV=nRT namount of substance n=PV/TR P=nRT/V I = temperature V=nRT/P T=PV/nR R=PV/nT 1. What pressure is required to contain 0.023 moles of nitrogen gas in a 4.2 L container at a temperature of 20.°C? 2. Oxygen gas is collected at a pressure of 123 kPa in a container which has a volume of 10.0 L. What temperature must be maintained on 0.500 moles of this gas in order to maintain this pressure? Express the temperature in degrees Celsius. 3. How many moles of chlorine gas would occupy a volume of 35.5 L at a pressure of 100.0 kPa and a temperature of 100. °C? After determining the number of moles,…arrow_forward1. The following conversion includes chemistry we have covered very recently, some chemistry from last term, and chemistry from the first chapter of this unit. Provide curly arrows and an explanation for this mechanism. Use the reagents in the order given. You do not need any other reagents. 1. NaOEt OEt 2.arrow_forwardCOOEt COOEt Step 1 Step 2 Step 3 COOEt COOEt COOH Step 6 OH Step 4 Step 7 (racemic) cyclizes under conditions (8) OTS Step 5 Step 8 ОН OH (racemic) Frontalin (racemic) Shown above are the steps in one of the several published syntheses of Frontalin, a pheromone of the western pine beetle. From the choices provided, show the reagents and conditions by which step 3 of this synthesis might be accomplished. List the reagent(s) in order that will accomplish this transformation. No more than 4 steps are required. List your answer as a single letter (single-step transformation) or a series of letters (multi-step transformation) with no commas separating them. For example, "ab" corresponds to: 1. Eto Na+ 2. NaOH, H₂O NOTE: The order in which you list your letters matters! Reagents: a. Eto Na* g. NaCN b. NaOH, H₂O h. SOCI₂ c. H3O+, heat i. (CH3)2CuLi, ether, -78°C d. LiAlH4 j. H₂O e. p-TsCI, pyridine k. RCO3H f. Br I. H3O+ 1,024arrow_forward
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
 World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning





