
(a)
Interpretation:
The element that is highlighted in red in the periodic table is present in s area or p area has to be determined.
Concept Introduction:
Periodic law states that if the elements are arranged in increasing order of
Location of an element in a periodic table can be given by the period number and the group number. The horizontal row in a periodic table where the elements are present is known as Period. The vertical column in a periodic table where the elements are present is known as Group.
Chemical properties of the elements repeat themselves at regular intervals because of the electronic configuration. The elements that are present in a Group have similar chemical properties. This is because the outer-shell electronic configuration will be the same.
The periodic table has all the elements that can be distinguished based on the outer-shell electron. If the outer-shell electron is present in s subshell, then the elements are present in s area of periodic table. If the outer-shell electron is present in p subshell, then the elements are present in p area of periodic table. If the outer-shell electron is present in d subshell, then the elements are present in d area of periodic table. If the outer-shell electron is present in f subshell, then the elements are present in f area of periodic table.
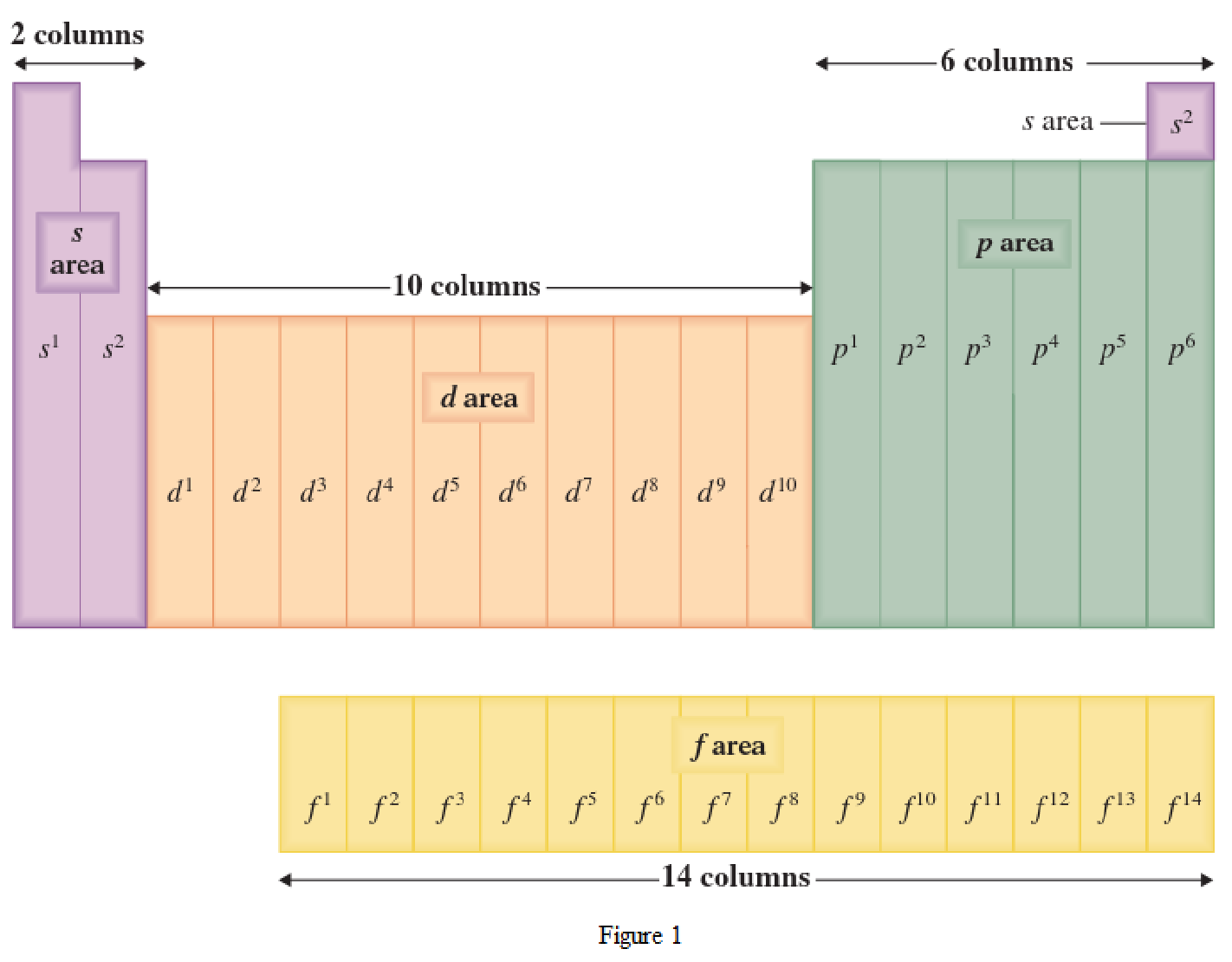
Distinguishing electron is the one that is the last electron added to the electronic configuration of an element when the electron subshells are filled in the order of increasing energy. This distinguishing electron determines the area of the element in the periodic table. This is because this only causes the element electronic configuration to differ from other elements.
(b)
Interpretation:
The element that is highlighted in green in the periodic table is present in d area or p area has to be determined.
Concept Introduction:
Periodic law states that if the elements are arranged in increasing order of atomic number, then the elements with similar chemical properties occur at regular intervals or periodic intervals. The elements are arranged in a periodic table in which the arrangement was based on the atomic number of the elements and the elements that have similar chemical properties are positioned in vertical columns.
Location of an element in a periodic table can be given by the period number and the group number. The horizontal row in a periodic table where the elements are present is known as Period. The vertical column in a periodic table where the elements are present is known as Group.
Chemical properties of the elements repeat themselves at regular intervals because of the electronic configuration. The elements that are present in a Group have similar chemical properties. This is because the outer-shell electronic configuration will be the same.
The periodic table has all the elements that can be distinguished based on the outer-shell electron. If the outer-shell electron is present in s subshell, then the elements are present in s area of periodic table. If the outer-shell electron is present in p subshell, then the elements are present in p area of periodic table. If the outer-shell electron is present in d subshell, then the elements are present in d area of periodic table. If the outer-shell electron is present in f subshell, then the elements are present in f area of periodic table.
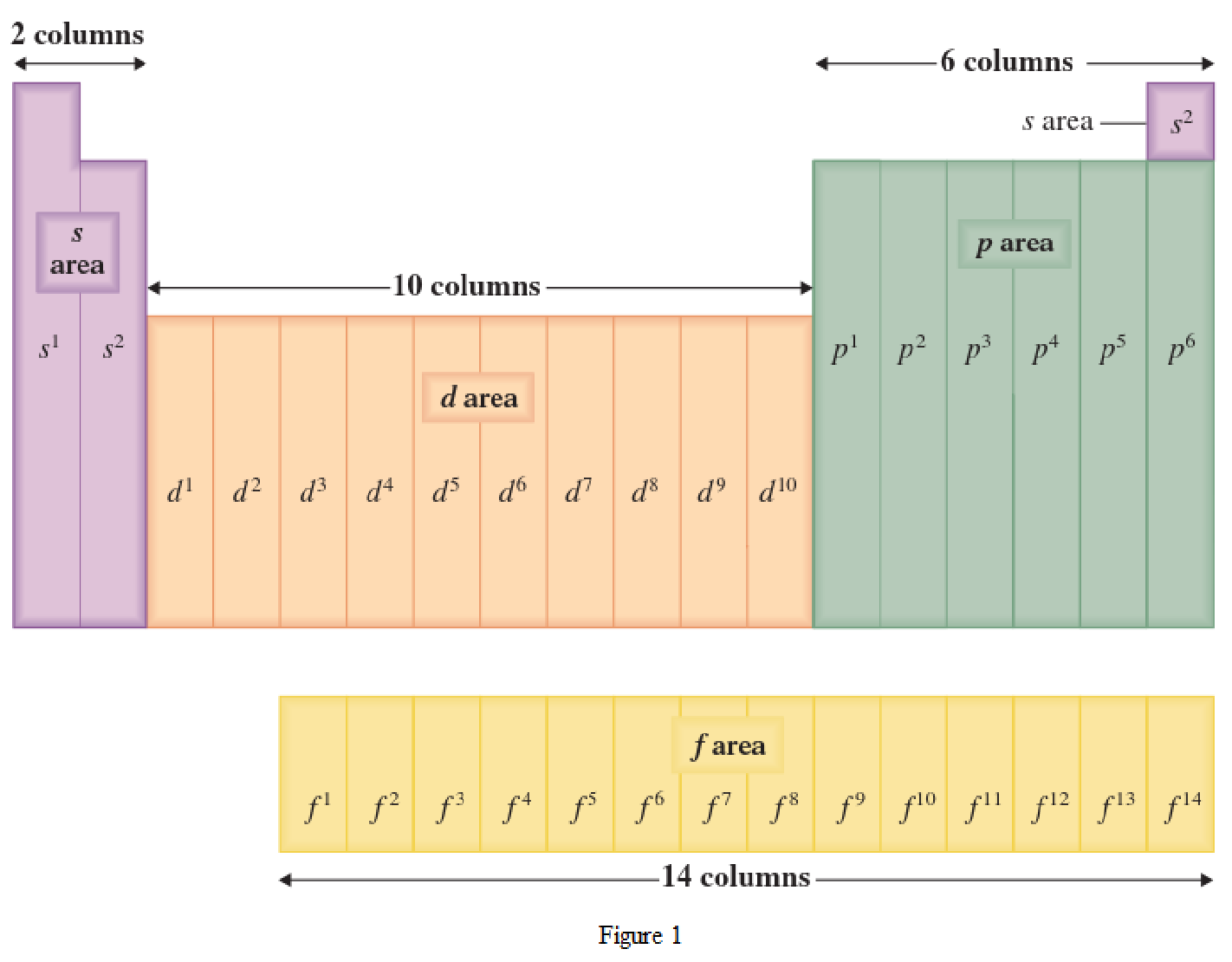
Distinguishing electron is the one that is the last electron added to the electronic configuration of an element when the electron subshells are filled in the order of increasing energy. This distinguishing electron determines the area of the element in the periodic table. This is because this only causes the element electronic configuration to differ from other elements.
(c)
Interpretation:
The element that is highlighted in yellow in the periodic table is a
Concept Introduction:
Periodic law states that if the elements are arranged in increasing order of atomic number, then the elements with similar chemical properties occur at regular intervals or periodic intervals. The elements are arranged in a periodic table in which the arrangement was based on the atomic number of the elements and the elements that have similar chemical properties are positioned in vertical columns.
Location of an element in a periodic table can be given by the period number and the group number. The horizontal row in a periodic table where the elements are present is known as Period. The vertical column in a periodic table where the elements are present is known as Group.
Chemical properties of the elements repeat themselves at regular intervals because of the electronic configuration. The elements that are present in a Group have similar chemical properties. This is because the outer-shell electronic configuration will be the same.
The periodic table has all the elements that can be distinguished based on the outer-shell electron. If the outer-shell electron is present in s subshell, then the elements are present in s area of periodic table. If the outer-shell electron is present in p subshell, then the elements are present in p area of periodic table. If the outer-shell electron is present in d subshell, then the elements are present in d area of periodic table. If the outer-shell electron is present in f subshell, then the elements are present in f area of periodic table.
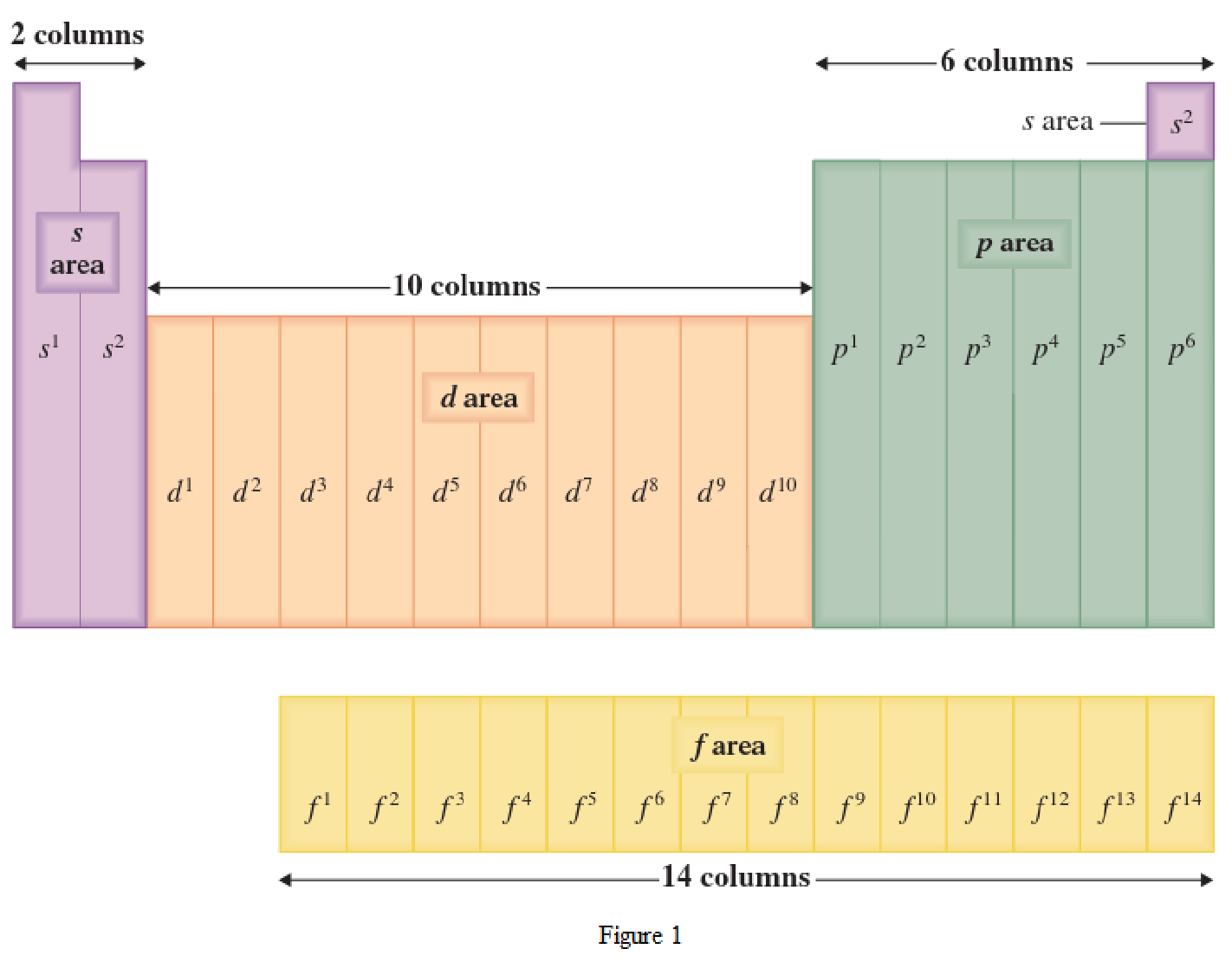
Distinguishing electron is the one that is the last electron added to the electronic configuration of an element when the electron subshells are filled in the order of increasing energy. This distinguishing electron determines the area of the element in the periodic table. This is because this only causes the element electronic configuration to differ from other elements.
(d)
Interpretation:
The element that is highlighted in blue in the periodic table is a
Concept Introduction:
Periodic law states that if the elements are arranged in increasing order of atomic number, then the elements with similar chemical properties occur at regular intervals or periodic intervals. The elements are arranged in a periodic table in which the arrangement was based on the atomic number of the elements and the elements that have similar chemical properties are positioned in vertical columns.
Location of an element in a periodic table can be given by the period number and the group number. The horizontal row in a periodic table where the elements are present is known as Period. The vertical column in a periodic table where the elements are present is known as Group.
Chemical properties of the elements repeat themselves at regular intervals because of the electronic configuration. The elements that are present in a Group have similar chemical properties. This is because the outer-shell electronic configuration will be the same.
The periodic table has all the elements that can be distinguished based on the outer-shell electron. If the outer-shell electron is present in s subshell, then the elements are present in s area of periodic table. If the outer-shell electron is present in p subshell, then the elements are present in p area of periodic table. If the outer-shell electron is present in d subshell, then the elements are present in d area of periodic table. If the outer-shell electron is present in f subshell, then the elements are present in f area of periodic table.
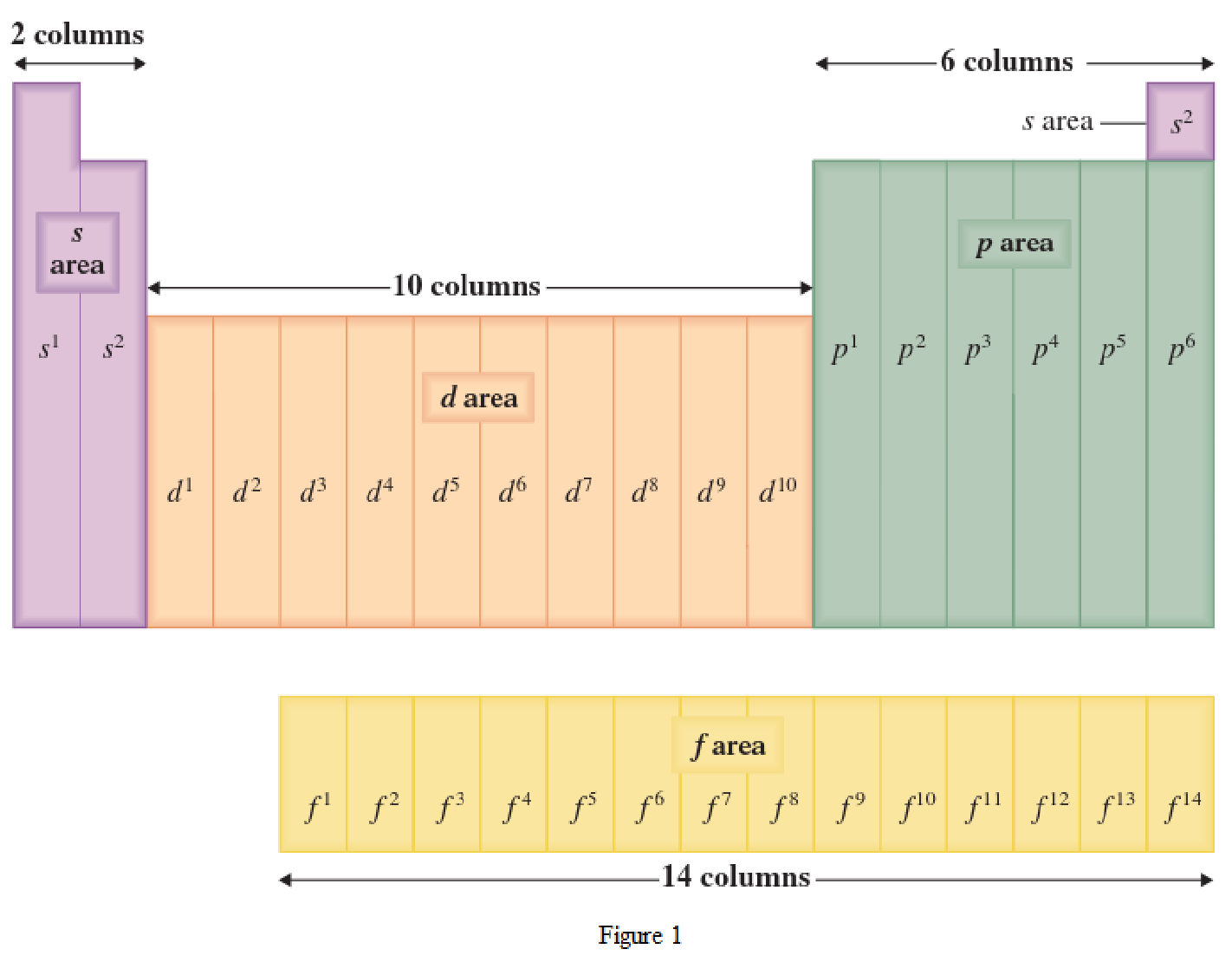
Distinguishing electron is the one that is the last electron added to the electronic configuration of an element when the electron subshells are filled in the order of increasing energy. This distinguishing electron determines the area of the element in the periodic table. This is because this only causes the element electronic configuration to differ from other elements.
Trending nowThis is a popular solution!

Chapter 3 Solutions
General, Organic, and Biological Chemistry Seventh Edition
- Assign the functional group bands on the IR spectra.arrow_forwardFind the pH of a 0.120 M solution of HNO2. Find the pH ignoring activity effects (i.e., the normal way). Find the pH in a solution of 0.050 M NaCl, including activityarrow_forwardPlease help me answer these three questions. Required info should be in data table.arrow_forward
- Draw the major organic substitution product or products for (2R,3S)-2-bromo-3-methylpentane reacting with the given nucleophile. Clearly drawn the stereochemistry, including a wedged bond, a dashed bond and two in-plane bonds at each stereogenic center. Omit any byproducts. Bri CH3CH2O- (conc.) Draw the major organic product or products.arrow_forwardTartaric acid (C4H6O6) is a diprotic weak acid. A sample of 875 mg tartaric acid are dissolved in 100 mL water and titrated with 0.994 M NaOH. How many mL of NaOH are needed to reach the first equivalence point? How many mL of NaOH are needed to reach the second equivalence point?arrow_forwardIncluding activity, calculate the solubility of Pb(IO3)2 in a matrix of 0.020 M Mg(NO3)2.arrow_forward
- Order the following series of compounds from highest to lowest reactivity to electrophilic aromatic substitution, explaining your answer: 2-nitrophenol, p-Toluidine, N-(4-methylphenyl)acetamide, 4-methylbenzonitrile, 4-(trifluoromethyl)benzonitrile.arrow_forwardOrdene la siguiente serie de compuestos de mayor a menor reactividad a la sustitución aromática electrofílica, explicando su respuesta: ácido bencenosulfónico, fluorobenceno, etilbenceno, clorobenceno, terc-butilbenceno, acetofenona.arrow_forwardCan I please get all final concentrations please!arrow_forward
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning





