
Concept explainers
Give the IUPAC names of each of the following:
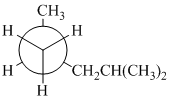
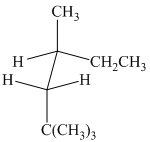
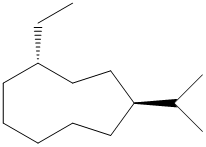
(a) (b) (c)
Interpretation:
IUPAC names of the given structures are to be determined.
Concept introduction:
When writing the IUPAC name, the longest continuous carbon chain is first determined. The parent chain is numbered such that the substituents get the lowest numbers.
The location of each substituent group is designated by an appropriate number and name. Prefixes are used if more than one substituents of the same type are present. The substituents are written in alphabetical order.
A cyclic ring hydrocarbon is designated by the prefix cyclo- which appears in front of the base name.
Answer to Problem 20P
Solution:
Explanation of Solution
(a)
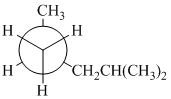
In the given Newman projection, the carbon atom at the front is a
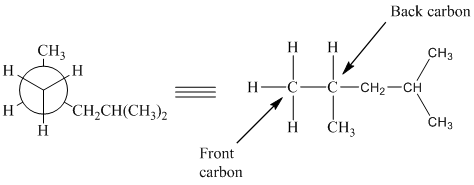
In this structure, the longest chain contains five carbon atoms, so the parent alkane is pentane. There are two methyl groups attached to this parent chain. The pentane chain is numbered such that these methyl groups get the lowest numbers.
The two methyl groups are attached to the carbon atoms
(b)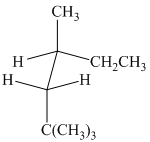
The given sawhorse projection is converted to a skeletal structure as follows:
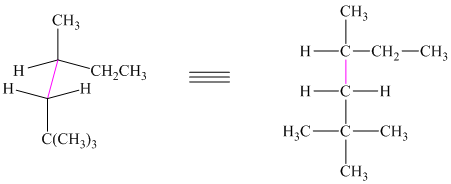
In this structure, the longest chain contains six carbon atoms, so the parent alkane is hexane. There are three methyl groups attached to the hexane chain. The chain is numbered such that the carbon atoms attached to these three methyl groups get the lowest numbers.
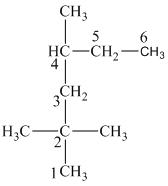
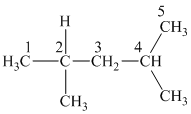
Two methyl groups are attached to
(c)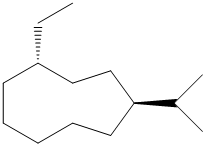
In the given structure, there are nine carbon atoms in the ring. So the name of parent alkane is cyclononane. The ring is numbered such that the two substituents get the lowest numbers.
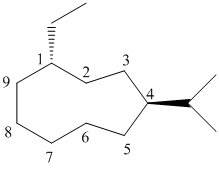
There is an ethyl group and an isopropyl group attached to the cyclononane ring. These two groups are trans to each other. Hence the ring is numbered such that the ethyl group gets the lower number since it comes first alphabetically. The IUPAC name of the compound is
Want to see more full solutions like this?
Chapter 3 Solutions
Organic Chemistry - Standalone book
- QUESTION: Fill out the answers to the empty green boxes attached in the image. *Ensure you all incorporate all 27 values (per column)*arrow_forwardYou need to make a buffer by dissolving benzoic acid and sodium benzoate in water. What is the mass of benzoic acid that you would weigh out, in mg, to create 50 mL of a buffer at pH = 4.7 that will change pH no more than 0.10 units with the addition of 0.001 moles of acid or base? Enter just the answer without the units (mg) - just the number will do!arrow_forwardDraw the formula for 3-isopropylcyclopentane-1-carbonyl chloride.arrow_forward
- QUESTION: Fill out the answers to the empty green boxes attached in the image. *Ensure you all incorporate all 27 values (per column)*arrow_forwardComplete the following reactions- hand written pleasearrow_forwardGive the organic product: O A O B Ос ○ D -NH–CH3 + CH3 CH3 NEN C ? A CH3 CH3 NH- CH3 B CH3 CH3 N=N- C CH3 CH3 N=NNH CH3 D CH3 N=N CH3 NHCH3 LNH CHOarrow_forward
- Finish the reaction- hand written pleasearrow_forwardGive the organic products: (benzyne) Br ? CH3 + K* :NH, liq NH3 HINT: Two products are formed. Each is a substituted aniline; they are isomers of each other. NH2 II I H₂N. CH3 CH3 III Select one: ○ A. I and II ○ B. I and III O C. I and IV O D. II and III O E. III and IV H₂N CH3 IV CH₂-NH2arrow_forwardPredict the major products of this organic reaction: HBr (1 equiv) cold ? Some important notes: • Draw the major product, or products, of this reaction in the drawing area below. • You can draw the products in any arrangement you like. • Pay careful attention to the reaction conditions, and only include the major products. • Be sure to use wedge and dash bonds when necessary, for example to distinguish between major products that are enantiomers. • Note that there is only 1 equivalent of HBr reactant, so you need not consider the case of multiple additions. Erase something Explanation Check 2025 McGraw Hill LLC. All Rights Reserved. Terarrow_forward
- Q14. Fill this chart: (please refer to ppt notes/browser to answer these questions) What alcohol is also called wood alcohol? What is the common name of ethanol? Draw the structure of phenol and thiophene? Are bigger chain alcohol like heptanol and octanol are soluble or insoluble in water and explain it ? Are ethers soluble or insoluble in water? What suffix and prefix are used for alcohol while naming alcohol and ether? What the process called when we add water to any alkene to make alcohol? Q16. Draw the diagram of following aromatic compound (practice from previous module) Aniline Phenol Benzoic acid Methyl benzoate Q17. a. Write the oxidation reactions for the 2 propanol. b. Write the oxidation reaction of the ethanol.arrow_forwardQuestion 11 of 18 (1 point) Question Attempt: 3 of How many signals do you expect in the 'H NMR spectrum for this molecule? Br Br Write the answer below. Also, in each of the drawing areas below is a copy of the molecule, with Hs shown. In each copy, one of the H atoms is colored red. Highlight in red all other H atoms that would contribute to the same signal as the H already highlighted red. Note for advanced students: In this question, any multiplet is counted as one signal. Number of signals in the 'H NMR spectrum. 1 For the molecule in the top drawing area, highlight in red any other H atoms that will contribute to the same signal as the H atom already highlighted red. If no other H atoms will contribute, check the box at right. No additional Hs to color in top molecule Check For the molecule in the bottom drawing area, highlight in red any other H atoms that will contribute to the same signal as the H atom already highlighted red. If no other H atoms will contribute, check the box…arrow_forwardOrganic Chemistry Esterification reactions 1. Write the steps to prepare ester. 2. Write complete reaction of ethanol and acetic acid to make ester. 3. What does ester smell like? What are the uses of ester. 4. What the role of sulfuric acid in the esterification reactionarrow_forward

 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning

