
To review:
The differences between the properties of water (H2O), ammonia (NH3), and methane (CH4), based on the hydrogen bonding in their structure. The heat of fusion for water, ammonia, and methane is given as 6.01, 5.66, and 0.94 kJ/mol, respectively. Also, determine increase or decrease in the density of ammonia in the form of ice (if generated), as compared to liquid ammonia.
Introduction:
The molecular mass of water, ammonia, and methane is almost equal, and all of them show tetrahedral geometry. The heat of fusion is the highest in water, decreases in ammonia, and is the least in methane. Water has a highly polar structure. The three molecules (water, ammonia, and methane) are sp3 hybridized and are tetrahedral instructure. However, there is a difference in the number of lone pairs in them, leading to an overall different geometry and different physical properties.
Explanation of Solution
Water has two hydrogen atom, shich are covalently bonded to one oxygen atom. The oxygen is sp3 hybridized. Due to the presence of twolone pairs, this molecule has a bent geometry. As a whole, a water molecule is polar and acts as a dipole. The electronegativity of oxygen is more than that of hydrogen, and thus, it bears a partial negative charge, whereas two hydrogen atoms beara partial positive charge. The hydrogen atoms in asingle water molecule are electrondeficient, and thus, tend to be attracted toward the oxygen atom of another water molecule. Thus, hydrogen bonds (intermolecular)act as bridges between neighboringwater molecules. One water molecule can form hydrogen bonds with four otherwater molecules.
The structures of thethreemolecules can be represented as
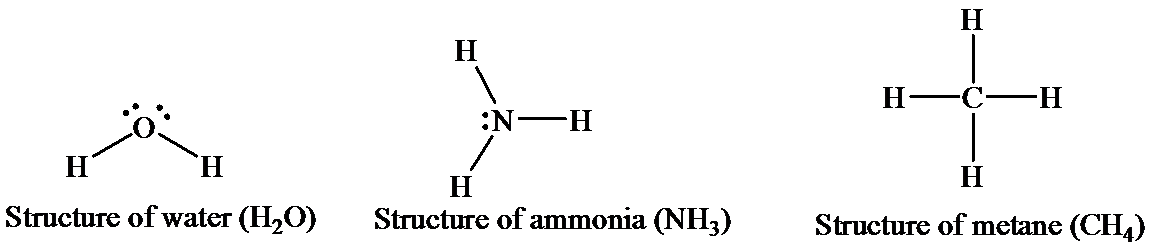
An ammoniamoleculehasonelone pair (unshared electron) ofa nitrogenatom and has a trigonal pyramidal structure. There is limited hydrogen bonding (intramolecular)in case of ammoniaasnitrogen has onlyone lone pair available.
Methane exists in a gaseous statethat has one carbon atom bonded with four hydrogen atoms. The central atom, that is, carbon, forms covalent bonds with four hydrogen atoms by the sharing of electrons. This sharing completes the outer shell of both the carbon and the hydrogen atoms. The four hydrogen atoms give an overall tetrahedral shape to the molecule. No hydrogen bonds are involved in methane as there is no highly electronegative element to form a bond with hydrogen.
A hydrogen bondhaslower bond strength thana covalent bond, thus, it is weaker. However, when a largenumber of intermolecular bonds are formed, they are quite strong. This explains the differences in the heat of fusion (energy required to melt ice by breaking bonds) for water, ammonia, and methane. Due to a highernumber of hydrogen bonds, physical propertie, such asmelting point, boiling point, heat capacity, the heat of fusion, surface tensio, nnd heat of vaporization, have higher values in wate, rs compared to that of methane and ammonia.
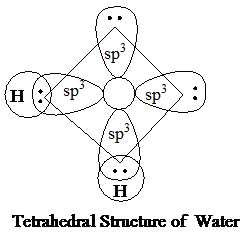


In ammonia, the density of the solid state (ice), if generated, is expected to be more than that of liquid ammonia. This is because the hydrogen bonding between different ammonia molecules will not be as extensive as in case of water insolid state (open cage structure in ice). This is due to limited hydrogen bonding of nitrogen. Thus, as a general case, the solid form of ammonia will beless denser than its liquid form.
Thus, it can be concluded that water, ammonia, and methane molecules are tetrahedral in structure, but differ in their overall geometry and physical properties due to thenumber of lone pairs in them and hydrogen bonding. The heat of fusion is the highest for water moleculesbecauseofstrong intermolecular hydrogen bonds. Also, ammonia ice will not form a cage-like structure (as in water), and thus, its density is expected to be lesser than that of liquid ammonia.
Want to see more full solutions like this?
Chapter 3 Solutions
Biochemistry: The Molecular Basis of Life
- Br Mg, ether 1. HCHO (formaldehyde) 2. H+, H₂O PCC 1. NH3, HCN ? (pyridinium chlorochromate) 2. H2O, HCI 11. Which one of the following compounds is the major organic product of the series of reactions shown above? Ph. Ph. OH NH2₂ A Ph. Ή NH2 B OH Ph Η Ph OH NH2 NH2₂ NH₂ C D Earrow_forwardB A 6. Which ONE of the labeled bonds in the tripeptide on the right is a peptide bond: H₂N N 'N' OH C H A, B, C, D or E? HN E OHarrow_forwardQuestions 8-9 are 0.4 points each. The next two questions relate to the peptide whose structure is shown here. To answer these questions, you should look at a table of H2N/.. amino acid structures. You don't have to memorize the structures of the amino acids. IZ 8. What is the N-terminal amino acid of this peptide? A) proline B) aspartic acid C) threonine 9. What is the C-terminal amino acid of this peptide? A) proline B) aspartic acid C) threonine N OH D) valine E) leucine D) valine E) leucine NH "OH OHarrow_forward
- 7. What is the correct name of the following tripeptide? A) Ile-Met-Ser B) Leu-Cys-Thr C) Val-Cys-Ser D) Ser-Cys-Leu E) Leu-Cys-Ser H₂N!!!!! N H ΖΙ .SH SF H IN OH OHarrow_forwardPlease draw out the following metabolic pathways: (Metabolic Map) Mitochondrion: TCA Cycle & GNG, Electron Transport, ATP Synthase, Lipolysis, Shuttle Systems Cytoplasm: Glycolysis & GNG, PPP (Pentose Phosphate Pathway), Glycogen, Lipogenesis, Transporters and Amino Acids Control: Cori/ Glc-Ala cycles, Insulin/Glucagon Reg, Local/Long Distance Regulation, Pools Used Correctlyarrow_forwardPlease help provide me an insight of what to draw for the following metabolic pathways: (Metabolic Map) Mitochondrion: TCA Cycle & GNG, Electron Transport, ATP Synthase, Lipolysis, Shuttle Systems Cytoplasm: Glycolysis & GNG, PPP (Pentose Phosphate Pathway), Glycogen, Lipogenesis, Transporters and Amino Acids Control: Cori/ Glc-Ala cycles, Insulin/Glucagon Reg, Local/Long Distance Regulation, Pools Used Correctlyarrow_forward
- f. The genetic code is given below, along with a short strand of template DNA. Write the protein segment that would form from this DNA. 5'-A-T-G-G-C-T-A-G-G-T-A-A-C-C-T-G-C-A-T-T-A-G-3' Table 4.5 The genetic code First Position Second Position (5' end) U C A G Third Position (3' end) Phe Ser Tyr Cys U Phe Ser Tyr Cys Leu Ser Stop Stop Leu Ser Stop Trp UCAG Leu Pro His Arg His Arg C Leu Pro Gln Arg Pro Leu Gin Arg Pro Leu Ser Asn Thr lle Ser Asn Thr lle Arg A Thr Lys UCAG UCAC G lle Arg Thr Lys Met Gly Asp Ala Val Gly Asp Ala Val Gly G Glu Ala UCAC Val Gly Glu Ala Val Note: This table identifies the amino acid encoded by each triplet. For example, the codon 5'-AUG-3' on mRNA specifies methionine, whereas CAU specifies histidine. UAA, UAG, and UGA are termination signals. AUG is part of the initiation signal, in addition to coding for internal methionine residues. Table 4.5 Biochemistry, Seventh Edition 2012 W. H. Freeman and Company B eviation: does it play abbreviation:arrow_forwardAnswer all of the questions please draw structures for major productarrow_forwardfor glycolysis and the citric acid cycle below, show where ATP, NADH and FADH are used or formed. Show on the diagram the points where at least three other metabolic pathways intersect with these two.arrow_forward
 Principles Of Radiographic Imaging: An Art And A ...Health & NutritionISBN:9781337711067Author:Richard R. Carlton, Arlene M. Adler, Vesna BalacPublisher:Cengage Learning
Principles Of Radiographic Imaging: An Art And A ...Health & NutritionISBN:9781337711067Author:Richard R. Carlton, Arlene M. Adler, Vesna BalacPublisher:Cengage Learning Biology (MindTap Course List)BiologyISBN:9781337392938Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. BergPublisher:Cengage Learning
Biology (MindTap Course List)BiologyISBN:9781337392938Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. BergPublisher:Cengage Learning
 Anatomy & PhysiologyBiologyISBN:9781938168130Author:Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark WomblePublisher:OpenStax College
Anatomy & PhysiologyBiologyISBN:9781938168130Author:Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark WomblePublisher:OpenStax College





