
Concept explainers
Refer to the color scheme given Figure 3-3, and give the molecular formulas for molecules whose ball-and-stick models are given here.
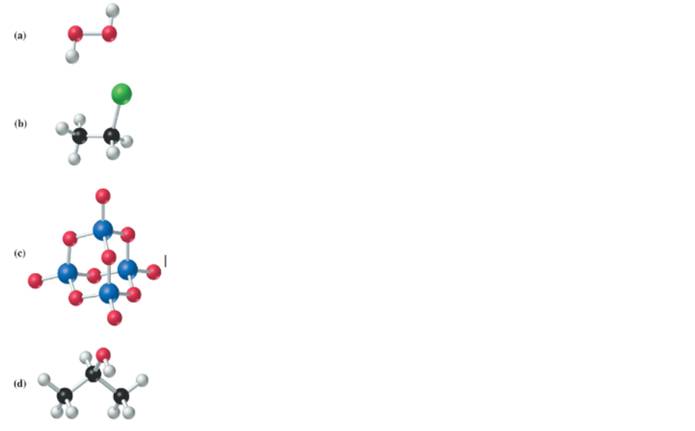
(a)
Interpretation:
The molecular formula for the given ball and stick model of the molecule needs to be determined.
Concept introduction:
The ball and stick model of a chemical substance is defined as a molecular model that displays the 3-D position of the atoms and bonds present between them. In the ball and stick model, the atoms are represented as balls or sphere where different atoms are shown by different colors and bonds are represented by sticks drawn between the atoms. The double and triple bonds are shown by drawing 2 and 3 sticks (curved rod) respectively between the atoms.
Answer to Problem 1E
Explanation of Solution
The given structure is as follows:
According to the color scheme given in figure 3.3 in the book, oxygen atom is represented by red sphere and hydrogen atom by white sphere. Thus, there are 2 oxygen atoms bonded together with single bond and 1 hydrogen atom each.
The molecular formula of the molecule will be
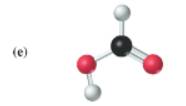
(b)
Interpretation:
The molecular formula for the given ball and stick model of the molecule needs to be determined.
Concept introduction:
The ball and stick model of a chemical substance is defined as a molecular model that displays the 3-D position of the atoms and bonds present between them. In the ball and stick model, the atoms are represented as balls or sphere where different atoms are shown by different colors and bonds are represented by sticks drawn between the atoms. The double and triple bonds are shown by drawing 2 and 3 sticks (curved rod) respectively between the atoms.
Answer to Problem 1E
Explanation of Solution
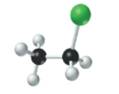
The given structure is as follows:
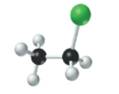
According to the color scheme given in figure 3.3 in the book, carbon atom is represented by black sphere, hydrogen atom by white sphere and chlorine atom by dark green sphere. Thus, there are 2 carbon atoms bonded together. One carbon atom is bonded with 3 hydrogen atoms and other with 2 hydrogen atoms and 1 chlorine atom.
The molecular formula of the molecule will be
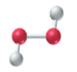
(c)
Interpretation:
The molecular formula for the given ball and stick model of the molecule needs to be determined.
Concept introduction:
The ball and stick model of a chemical substance is defined as a molecular model that displays the 3-D position of the atoms and bonds present between them. In the ball and stick model, the atoms are represented as balls or sphere where different atoms are shown by different colors and bonds are represented by sticks drawn between the atoms. The double and triple bonds are shown by drawing 2 and 3 sticks (curved rod) respectively between the atoms.
Answer to Problem 1E
Explanation of Solution
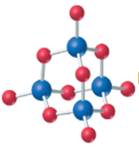
The given structure is as follows:
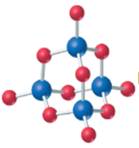
According to the color scheme given in figure 3.3 in the book, phosphorus atom is represented by dark blue sphere and oxygen atom with red sphere. Thus, there are 4 phosphorus atoms and 10 oxygen atoms. The molecular formula of the molecule will be
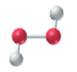
(d)
Interpretation:
The molecular formula for the given ball and stick model of the molecule needs to be determined.
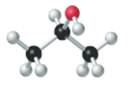
Concept introduction:
The ball and stick model of a chemical substance is defined as a molecular model that displays the 3-D position of the atoms and bonds present between them. In the ball and stick model, the atoms are represented as balls or sphere where different atoms are shown by different colors and bonds are represented by sticks drawn between the atoms. The double and triple bonds are shown by drawing 2 and 3 sticks (curved rod) respectively between the atoms.
Answer to Problem 1E
Explanation of Solution
The given structure is as follows:
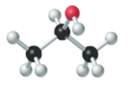
According to the color scheme given in figure 3.3 in the book, carbon atom is represented by black sphere, hydrogen atom by white sphere and oxygen atom by red sphere. Thus, there are 3 carbon atoms. The 2 carbon atoms are attached with 3 hydrogen atoms each and 1 carbon atom is attached with 2 hydrogen atoms and 1oxygen atom. The molecular formula of the molecule will be
(e)
Interpretation:
The molecular formula for the given ball and stick model of the molecule needs to be determined.
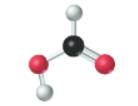 Concept introduction:
Concept introduction:
The ball and stick model of a chemical substance is defined as a molecular model that displays the 3-D position of the atoms and bonds present between them. In the ball and stick model, the atoms are represented as balls or sphere where different atoms are shown by different colors and bonds are represented by sticks drawn between the atoms. The double and triple bonds are shown by drawing 2 and 3 sticks (curved rod) respectively between the atoms.
Answer to Problem 1E
Explanation of Solution
The given molecule is as follows:
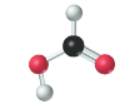
According to the color scheme given in figure 3.3 in the book, carbon atom is represented by black sphere, hydrogen atom by white sphere and oxygen atom by red sphere. The 2 curved rods between 1 carbon and 1 oxygen atom represents the double bond. Thus, there is 1 carbon atom bonded with 1 oxygen atom via double bond, 1 hydrogen atom via single bonds and 1 oxygen atom via single bond which is further attached with another hydrogen atom. The molecular formula of the molecule will be
Want to see more full solutions like this?
Chapter 3 Solutions
General Chemistry: Principles and Modern Applications (11th Edition)
Additional Science Textbook Solutions
Brock Biology of Microorganisms (15th Edition)
HUMAN ANATOMY
Laboratory Experiments in Microbiology (12th Edition) (What's New in Microbiology)
Organic Chemistry
Human Biology: Concepts and Current Issues (8th Edition)
General, Organic, and Biological Chemistry - 4th edition
- Label all absorptions over 1500 cm-1. Please be specific and mark IR if needed for explanation. Compound OH sp^3 C-H C=O C-O Triglyceridearrow_forwardIdentify the intermediate that is INITIALLY formed in a saponification reaction (hydrolysis of an ester). III -OH H₂O HO OH HO O || A B C III D IV IVarrow_forwardHelp me answer this practice sheet I found for an answer guidearrow_forward
- show the retrosynthesis of this molecule step by step starting with 1,3-dimethoxy benzene H3CO OH OH OCH 3arrow_forwardConsider the reaction of a propanoate ester with hydroxide ion shown below. A series of four alcohol leaving groups were tested to determine which would be the best leaving group. Based on the pKa values of the alcohols, predict which alcohol would produce the fastest hydrolysis reaction. HO FOR A Alcohol I, pKa =16.0 B Alcohol II, pKa =10.0 C Alcohol III, pKa = 7.2 + ROH D Alcohol IV, pKa = 6.6arrow_forwardCurved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. :0: NaOH, H₂O 00:4 Na O heat NaO Select to Add Arrows Select to Add Arrows :0: Na a NaOH, H2O :0: NaOH, H2O heat heat Na ONH Select to Add Arrowsarrow_forward
- Curved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. H CH3NH3+ :0: :0: HO CH3NH2 HH iSelect to Add Arrows i Select to Add Arrows i HH CH3NH3+ CH3NH2 Select to Add Arrows i CH3NH3 CH3NH2 ايكدا HH Select to Add Arrowsarrow_forwardThe reaction is carried out with gases: A → B + C at 300 K. The total pressure is measured as a function of time (table). If the reaction order is 2, calculate the rate or kinetic constant k (in mol-1 L s¹) Ptotal (atm) 492 676 760 808 861 t(s) 0 600 1200 1800 3000arrow_forwardcan someone give a description of this NMR including whether its a triplt singlet doublet where the peak is around at ppm and what functional group it representsarrow_forward
- 1. Determine the relationship between the following molecules as identical, diastereomers, or enantiomers (6 points, 2 points each). OH OH OH A-A OH HOT HO- ACHN and HO- ACHN OH HO HO ° OH and OH OH SH and ...SHarrow_forward20,0 Complete the electron pushing mechanism to y drawing the necomery unicaciones and carved on for Step 1: Add curved arms for the tint step, traiment with NalilĻ. The Nation 458 Step 2: Added for the second step, inalment with), how the "counterion bar Step 3: Daw the products of the last simplom organic and one incoganic spacient, including all nonbondingarrow_forwardplease provide the structure for this problem, thank you!arrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning


