
Introduction to General, Organic and Biochemistry
12th Edition
ISBN: 9780357119303
Author: Bettelheim, Frederick A., Brown, William H., Campbell, Mary K., FARRELL, Shawn O., Torres, Omar
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 3, Problem 114P
Consider the structure of lipoic acid shown below, a growth factor for many bacteria and protozoa that functions as an essential component of enzymes involved in human
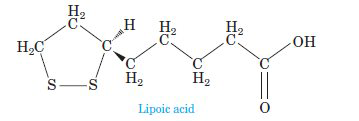
(a) Identify the various types of geometries present in each central atom denoted by an arrow using VSEPR theory.
(b) Determine the various relative bond angles associated with each central atom denoted by an arrow using VSEPR theory.
(c) What is the most polar bond in lipoic acid?
(d) Would you predict lipoic acid to be polar or nonpolar?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Part 1. Draw monomer units of the following products and draw their reaction mechanism
1) Bakelite like polymer
Using: Resorcinol + NaOH + Formalin
2) Polyester fiber
Using a) pthalic anhydride + anhydrous sodium acetate + ethylene glycol
B)pthalic anhydride + anhydrous sodium acetate + glycerol
3) Temporary cross-linked polymer
Using: 4% polyvinyl alcohol+ methyl red + 4% sodium borate
Using the table of Reactants and Products provided provide the correct letter that
corresponds with the Carboxylic acid that is formed in the reaction below.
6 M NaOH
Acid-workup
WRITE THE CORRECT LETTER ONLY DO NOT WRITE EXTRA WORDS OR
PHRASES
A)
Pool of Reagents for Part B
CI
B)
OH
C)
E)
CI
J)
racemic
F)
K)
OH
N)
OH
P)
G)
OH
D)
HO
H)
L)
M)
HO
Q)
R)
CI
A
In the table below, the exact chemical structures for Methyl salicylate can be
represented by the letter
WRITE THE CORRECT LETTER ONLY DO NOT WRITE EXTRA WORDS OR
PHRASES
CI
B)
A)
E)
Cl
racemic
F)
J)
CI
K)
N)
OH
P)
Pool of Reagents for Part B
OH
OH
G)
L)
OH
D)
HO
H)
M)
HO
Q)
R)
CI
Chapter 3 Solutions
Introduction to General, Organic and Biochemistry
Ch. 3.1 - Problem 3-1 Show how the following chemical...Ch. 3.3 - Problem 3-2 Judging from their relative positions...Ch. 3.4 - Problem 3-3 Write the formulas for the ionic...Ch. 3.5 - Problem 3-4 Name these binary ionic compounds: (a)...Ch. 3.5 - Prob. 3.5QCCh. 3.5 - Problem 3-6 Give each binary compound a systematic...Ch. 3.5 - Problem 3-7 Name these ionic compounds, each of...Ch. 3.6 - Prob. 3.8QCCh. 3.6 - Prob. 3.9QCCh. 3.6 - Prob. 3.10QC
Ch. 3.6 - Prob. 3.11QCCh. 3.7 - Prob. 3.12QCCh. 3.8 - Prob. 3.13QCCh. 3.8 - Prob. 3.14QCCh. 3.9 - Problem 3-15 Predict all bond angles for these...Ch. 3.10 - Problem 3-16 Which of these molecules are polar?...Ch. 3 - 3-17 Answer true or false. (a) The octet rule...Ch. 3 - 3-18 How many electrons must each atom gain or...Ch. 3 - 3-19 Show how each chemical change obeys the octet...Ch. 3 - 3-20 Show how each chemical change obeys the octet...Ch. 3 - 3-21 Write the formula for the most stable ion...Ch. 3 - 3-22 Why is Li- not a stable ion?Ch. 3 - 3-23 Predict which ions are stable: (a) (b) (c)...Ch. 3 - 3-24 Predict which ions are stable: (a) Br2- (b)...Ch. 3 - 3-25 Why are carbon and silicon reluctant to form...Ch. 3 - 3-26 Table 3-2 shows the following ions of copper:...Ch. 3 - 3-27 Answer true or false. (a) For Group lA and...Ch. 3 - 3-28 Name each polyatomic ion. (a) HCO3- (b) NO2-...Ch. 3 - 3-29 Answer true or false. (a) According to the...Ch. 3 - Prob. 14PCh. 3 - 3-31 Why does electronegativity generally increase...Ch. 3 - 3-32 Judging from their relative positions in the...Ch. 3 - Prob. 17PCh. 3 - 3-34 Which of these bonds is the most polar? The...Ch. 3 - 3-35 Classify each bond as nonpolar covalent,...Ch. 3 - 3-36 Classify each bond as nonpolar covalent,...Ch. 3 - 3-37 Answer true or false. (a) An ionic bond is...Ch. 3 - 3-38 Complete the chart by writing formulas for...Ch. 3 - 3-39 Write a formula for the ionic compound formed...Ch. 3 - Prob. 24PCh. 3 - 3-41 Describe the structure of sodium chloride in...Ch. 3 - 3-42 What is the charge on each ion in these...Ch. 3 - 3-43 Write the formula for the compound formed...Ch. 3 - 3-44 Write the formula for the ionic compound...Ch. 3 - 3-45 Which formulas are not correct? For each that...Ch. 3 - 3-46 Which formulas are not correct? For each that...Ch. 3 - 3-47 Answer true or false. (a) The name of a...Ch. 3 - 3-48 Potassium chloride and potassium bicarbonate...Ch. 3 - Prob. 33PCh. 3 - 3-50 Name the polyatomic ion(s) in each compound....Ch. 3 - 3-51 Write the formulas for the ions present in...Ch. 3 - Prob. 36PCh. 3 - 3-53 Write formulas for the following ionic...Ch. 3 - 3-54 Write formulas for the following ionic...Ch. 3 - Prob. 39PCh. 3 - 3-56 How many covalent bonds are normally formed...Ch. 3 - 3-57 What is: (a) A single bond? (b) A double...Ch. 3 - 3-58 In Section 2-3B, we saw that there are seven...Ch. 3 - Prob. 43PCh. 3 - Prob. 44PCh. 3 - Prob. 45PCh. 3 - Prob. 46PCh. 3 - 3-63 What is the difference between (a) a bromine...Ch. 3 - 3-64 Acetylene (C2H2), hydrogen cyanide (HCN), and...Ch. 3 - Prob. 49PCh. 3 - 3-66 Why can’t second-row elements have more than...Ch. 3 - 3-67 Why does nitrogen have three bonds and one...Ch. 3 - 3-68 Draw a Lewis structure of a covalent compound...Ch. 3 - Prob. 53PCh. 3 - 3-70 Draw a Lewis structure of a covalent compound...Ch. 3 - Prob. 55PCh. 3 - Prob. 56PCh. 3 - Prob. 57PCh. 3 - 3-74 Answer true or false. (a) A binary covalent...Ch. 3 - Prob. 59PCh. 3 - Prob. 60PCh. 3 - 3-77 Ozone, O3, is an unstable blue gas with a...Ch. 3 - 3-78 Nitrous oxide, N20, laughing gas, is a...Ch. 3 - 3-79 Answer true or false. (a) The letters VSEPR...Ch. 3 - Prob. 64PCh. 3 - Prob. 65PCh. 3 - 3-82 Hydrogen and nitrogen combine in different...Ch. 3 - Prob. 67PCh. 3 - Prob. 68PCh. 3 - Prob. 69PCh. 3 - Prob. 70PCh. 3 - 3-87 Consider the molecule boron trffluoride, BF3....Ch. 3 - Prob. 72PCh. 3 - 3-89 Is it possible for a molecule to have no...Ch. 3 - Prob. 74PCh. 3 - Prob. 75PCh. 3 - Prob. 76PCh. 3 - Prob. 77PCh. 3 - Prob. 78PCh. 3 - Prob. 79PCh. 3 - Prob. 80PCh. 3 - Prob. 81PCh. 3 - Prob. 82PCh. 3 - 3-99 Knowing what you do about covalent bonding in...Ch. 3 - Prob. 84PCh. 3 - Prob. 85PCh. 3 - Prob. 86PCh. 3 - Prob. 87PCh. 3 - Prob. 88PCh. 3 - 3-105 Consider the structure of Vitamin E shown...Ch. 3 - 3-106 Consider the structure of Penicillin G shown...Ch. 3 - 3-107 Ephedrine, a molecule at one time found in...Ch. 3 - Prob. 92PCh. 3 - 3-109 Until several years ago, the two...Ch. 3 - 3-110 Name and write the formula for the fluorine...Ch. 3 - Prob. 95PCh. 3 - Prob. 96PCh. 3 - Prob. 97PCh. 3 - Prob. 98PCh. 3 - Prob. 99PCh. 3 - Prob. 100PCh. 3 - Prob. 101PCh. 3 - Prob. 102PCh. 3 - 3-119 Perchloroethylene, which is a liquid at room...Ch. 3 - 3-120 Vinyl chloride is the starting material for...Ch. 3 - 3-121 Tetrafluoroethylene is the starting material...Ch. 3 - 3-122 Some of the following structural formulas...Ch. 3 - 3-123 Sodium borohydride, NaBH4, has found wide...Ch. 3 - Prob. 108PCh. 3 - Prob. 109PCh. 3 - Prob. 110PCh. 3 - Prob. 111PCh. 3 - Prob. 112PCh. 3 - Consider the structure of Fluoxetine (or Prozac)...Ch. 3 - Consider the structure of lipoic acid shown below,...Ch. 3 - Prob. 115P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Draw the stepwise mechanism for the reactionsarrow_forwardPart I. a) Draw reaction mechanism for the transformations of benzophenone to benzopinacol to benzopinaco lone b) Pinacol (2,3-dimethyl, 1-3-butanediol) on treatment w/ acid gives a mixture of pina colone (3,3-dimethyl-2-butanone) and 2, 3-dimethyl - 1,3-butadiene. Give reasonable mechanism the formation of the products Forarrow_forward3. The explosive decomposition of 2 mole of TNT (2,4,6-trinitrotoluene) is shown below: Assume the C(s) is soot-basically atomic carbon (although it isn't actually atomic carbon in real life). 2 CH3 H NO2 NO2 3N2 (g)+7CO (g) + 5H₂O (g) + 7C (s) H a. Use bond dissociation energies to calculate how much AU is for this reaction in kJ/mol.arrow_forward
- Part I. Draw reaction mechanism for the transformations of benzophenone to benzopinacol to benzopinaco lone and answer the ff: Pinacol (2,3-dimethyl, 1-3-butanediol) on treatment w/ acid gives a mixture of pina colone and (3,3-dimethyl-2-butanone) 2,3-dimethyl-1,3-butadiene. Give reasonable mechanism the formation of the products Forarrow_forwardShow the mechanism for these reactionsarrow_forwardDraw the stepwise mechanismarrow_forward
- Draw a structural formula of the principal product formed when benzonitrile is treated with each reagent. (a) H₂O (one equivalent), H₂SO₄, heat (b) H₂O (excess), H₂SO₄, heat (c) NaOH, H₂O, heat (d) LiAlH4, then H₂Oarrow_forwardDraw the stepwise mechanism for the reactionsarrow_forwardDraw stepwise mechanismarrow_forward
- Part I. Draw reaction mechanism for the transformations of benzophenone to benzopinacol to benzopinaco lone and answer the ff: a) Give the major reason for the exposure of benzophenone al isopropyl alcohol (w/acid) to direct sunlight of pina colone Mechanism For b) Pinacol (2,3-dimethy 1, 1-3-butanediol) on treatment w/ acid gives a mixture (3,3-dimethyl-2-butanone) and 2, 3-dimethyl-1,3-butadiene. Give reasonable the formation of the productsarrow_forwardwhat are the Iupac names for each structurearrow_forwardWhat are the IUPAC Names of all the compounds in the picture?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
Stoichiometry - Chemistry for Massive Creatures: Crash Course Chemistry #6; Author: Crash Course;https://www.youtube.com/watch?v=UL1jmJaUkaQ;License: Standard YouTube License, CC-BY
Bonding (Ionic, Covalent & Metallic) - GCSE Chemistry; Author: Science Shorts;https://www.youtube.com/watch?v=p9MA6Od-zBA;License: Standard YouTube License, CC-BY
General Chemistry 1A. Lecture 12. Two Theories of Bonding.; Author: UCI Open;https://www.youtube.com/watch?v=dLTlL9Z1bh0;License: CC-BY