
PKG ORGANIC CHEMISTRY
5th Edition
ISBN: 9781259963667
Author: SMITH
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 29, Problem 29.65P
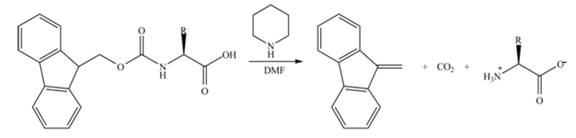
Draw the mechanism for the reaction that removes an Fmoc group from an amino acid under the following conditions:
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Neostigmine is an inhibitor of acetylcholinesterase. The enzyme attempts to catalyse the same reaction on neostigmine as it does with acetylcholine. However, a stable intermediate is formed which prevents completion of the process and which results in a molecule being covalently linked to the active site. Identify (draw) the stable intermediate and explain why it is stable.
An enzyme catalyzes the hydrolysis of an ester with a
certain activity, but this activity is lost in a 3 M urea
solution. What is the most likely explanation for the loss
of activity?
(A) Urea binds to the active site of the enzyme
competitively with the substrate.
(B) Urea causes the cleavage of the peptide bonds in the
enzyme.
(C) Urea causes the enzyme to denature and lose its
specific three-dimensional shape.
(D) Urea reacts with disulfide bonds in the enzyme.
lodoacetamide acts as an irreversible inhibitor for a few enzymes by reacting with the amino acid at the active site having the function group
-NH_2
-COOH
-SH
-OH
Chapter 29 Solutions
PKG ORGANIC CHEMISTRY
Ch. 29 - Prob. 29.1PCh. 29 - Problem 29.2
What form exists at the isoelectric...Ch. 29 - Problem 29.3
Explain why the of the group of an...Ch. 29 - Prob. 29.4PCh. 29 - Problem 29.5
What -halo carbonyl compound is...Ch. 29 - Problem 29.6
The enolate derived from diethyl...Ch. 29 - Problem 29.7
What amino acid is formed when is...Ch. 29 - Problem 29.8
What aldehyde is needed to synthesize...Ch. 29 - Problem 29.9
Draw the products of each...Ch. 29 - Prob. 29.10P
Ch. 29 - Prob. 29.11PCh. 29 - Prob. 29.12PCh. 29 - Problem 29.13
What alkene is needed to synthesize...Ch. 29 - Problem 29.14
Draw the structure of each peptide....Ch. 29 - Problem 29.15
Name each peptide using both the...Ch. 29 - Prob. 29.16PCh. 29 - Prob. 29.17PCh. 29 - Problem 29.18
Glutathione, a powerful antioxidant...Ch. 29 - Problem 29.19
Draw the structure of the...Ch. 29 - Problem 29.20
Give the amino acid sequence of an...Ch. 29 - a What products are formed when each peptide is...Ch. 29 - Prob. 29.22PCh. 29 - Devise a synthesis of each peptide from amino acid...Ch. 29 - Devise a synthesis of the following dipeptide from...Ch. 29 - Prob. 29.25PCh. 29 - Consider two molecules of a tetrapeptide composed...Ch. 29 - What types of stabilizing interactions exist...Ch. 29 - Prob. 29.28PCh. 29 - Draw the product formed when the following amino...Ch. 29 - With reference to the following peptide: a...Ch. 29 - Devise a synthesis of the following dipeptide from...Ch. 29 - Prob. 29.32PCh. 29 - Histidine is classified as a basic amino acid...Ch. 29 - Tryptophan is not classified as a basic amino acid...Ch. 29 - What is the structure of each amino acid at its...Ch. 29 - What is the predominant form of each of the...Ch. 29 - 29.37 What is the predominant form of each of the...Ch. 29 - a. Draw the structure of the tripeptide A–A–A, and...Ch. 29 - 29.39 Draw the organic products formed in each...Ch. 29 - 29.40 What alkyl halide is needed to synthesize...Ch. 29 - 29.41 Devise a synthesis of threonine from diethyl...Ch. 29 - 29.42 Devise a synthesis of each amino acid from...Ch. 29 - Prob. 29.43PCh. 29 - Prob. 29.44PCh. 29 - Prob. 29.45PCh. 29 - Prob. 29.46PCh. 29 - Prob. 29.47PCh. 29 - 29.48 Brucine is a poisonous alkaloid obtained...Ch. 29 - Prob. 29.49PCh. 29 - Prob. 29.50PCh. 29 - Draw the structure for each peptide: (a) Phe–Ala;...Ch. 29 - 29.52 For the tetrapeptide Asp–Arg–Val–Tyr:
a....Ch. 29 - Prob. 29.53PCh. 29 - Prob. 29.54PCh. 29 - 29.55 Draw the amino acids and peptide fragments...Ch. 29 - Prob. 29.56PCh. 29 - Prob. 29.57PCh. 29 - Prob. 29.58PCh. 29 - 29.59 An octapeptide contains the following amino...Ch. 29 - 29.60 Draw the organic products formed in each...Ch. 29 - Draw all the steps in the synthesis of each...Ch. 29 - 29.62 Write out the steps for the synthesis of...Ch. 29 - 29.63 Besides the Boc and Fmoc protecting groups...Ch. 29 - 29.64 Another method to form a peptide bond...Ch. 29 - 29.65 Draw the mechanism for the reaction that...Ch. 29 - 29.66 Which of the following amino acids are...Ch. 29 - 29.67 After the peptide chain of collagen has been...Ch. 29 - Prob. 29.68PCh. 29 - Prob. 29.69PCh. 29 - 29.70 The anti-obesity drug orlistat works by...Ch. 29 - Prob. 29.71P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Proteins that helps in increasing the rate of chemical reaction in the body * O(a) Enzymes O (b) Antibody O (c) Structural proteins O (d) Transport proteinsarrow_forwardWhat is the mechanism of action of the antibiotic drug Sulfanilamide in terms of enzyme inhibition?arrow_forwardWhich of the following amino acids can work as acid or base when present in the active site of an enzyme? (Note: Only R group would contribute to acid-base property) O Aspartate O Phenylalanine O Isoleucine O Glutaminearrow_forward
- The conversion of L-proline to D-proline is shown. The reaction below shows an enzyme-catalyzed reaction that converts from an L amino acid to D amino acid. What type of enzyme catalyzes this reaction? H H+ COOH L-Proline Isomerase Hydrolase Ligase Transferase COOH Transition state H Xco N COOH H D-Prolinearrow_forwardAsap pleasearrow_forwardPredict the product(s) obtained when each of the following compounds is treated with a mixture of nitric acid and sulfuric acid. Select all that apply. 18.41d * Your answer is incorrect.arrow_forward
- How prepare g-C3N4 from urea in laboratory?arrow_forwardSulfanilamide, a sulfur drug, acts as an antibiotic. Explain its mechanism of action in the context of enzyme inhibition. Methotrexate is used in cancer chemotherapy. Explain how this compound works by elaborating on the type of enzyme inhibition involved for its action.arrow_forward3. What is the product if the following peptide is hydrolyzed? H₂N O OHarrow_forward
- Give the name of the enzyme that will most likely catalyze each of the following reactions: O CH3 - CH2 - OH + NAD+ → CH3 – C – H + NADH + H+ Ans. ___________________________________________ CH3 – C – COOH + R – CH – COOH → CH3 – CH – COOH + R – C – COOH O NH2 NH2 O HO – C – CH – CH2 – C – OH → HO – C – CH = CH – C – OH + H2O O OH O O O CO2 + CH3 – C – C – OH + ATP → HO – C – CH2 – C – C – OH + ADP + Pi O O O O…arrow_forwardDraw the structure of the following molecule The amino acid phenylalanine at pH 1arrow_forwardBelow is given the active site of a protease including its substrate. SUBSTRATE R1-NH NH Ph NH OH N ENZYME (i) Which type of protease is this? (ii) Draw the same part of the enzyme at the intermediate stage of the enzyme catalyzed reaction when part of the substrate is covalently linked to the enzyme.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:9781305081079
Author:STOKER, H. Stephen (howard Stephen)
Publisher:Cengage Learning,


Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning
Biomolecules - Protein - Amino acids; Author: Tutorials Point (India) Ltd.;https://www.youtube.com/watch?v=ySNVPDHJ0ek;License: Standard YouTube License, CC-BY