
PKG ORGANIC CHEMISTRY
5th Edition
ISBN: 9781259963667
Author: SMITH
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 29, Problem 29.70P
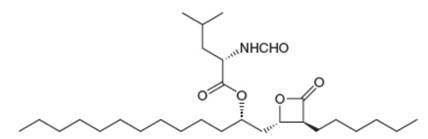
The anti-obesity drug orlistat works by irreversibly inhibiting pancreatic lipase, an enzyme responsible for the hydrolysis of triacylglycerols in the intestines, so they are excreted without
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Using reaction free energy to predict equilibrium composition
Consider the following equilibrium:
2NH3 (g) = N2 (g) +3H₂
—N2 (g) AGº = 34. kJ
Now suppose a reaction vessel is filled with 4.19 atm of ammonia (NH3) and 9.94 atm of nitrogen (N2) at 378. °C. Answer the following questions about this
system:
rise
Under these conditions, will the pressure of NH 3 tend to rise or fall?
☐ x10
fall
Х
Is it possible to reverse this tendency by adding H₂?
In other words, if you said the pressure of NH 3 will tend to rise, can that
be changed to a tendency to fall by adding H₂? Similarly, if you said the
pressure of NH3 will tend to fall, can that be changed to a tendency to
rise by adding H₂?
If you said the tendency can be reversed in the second question, calculate
the minimum pressure of H₂ needed to reverse it.
Round your answer to 2 significant digits.
yes
no
atm
00.
18
Ar
무ㅎ
?
Identifying the major species in weak acid or weak base equilibria
The preparations of two aqueous solutions are described in the table below. For each solution, write the chemical formulas of the major species present at
equilibrium. You can leave out water itself.
Write the chemical formulas of the species that will act as acids in the 'acids' row, the formulas of the species that will act as bases in the 'bases' row, and the
formulas of the species that will act as neither acids nor bases in the 'other' row.
You will find it useful to keep in mind that HF is a weak acid.
2.2 mol of NaOH is added to
1.0 L of a 1.4M HF
solution.
acids:
П
bases:
Х
other: ☐
ப
acids:
0.51 mol of KOH is added to
1.0 L of a solution that is
bases:
1.3M in both HF and NaF.
other: ☐
00.
18
Ar
Using reaction free energy to predict equilibrium composition
Consider the following equilibrium:
N2O4 (g) 2NO2 (g)
AG⁰ = 5.4 kJ
Now suppose a reaction vessel is filled with 1.68 atm of dinitrogen tetroxide (N204) at 148. °C. Answer the following questions about this system:
rise
Under these conditions, will the pressure of N2O4 tend to rise or fall?
x10
fall
Is it possible to reverse this tendency by adding NO2?
In other words, if you said the pressure of N2O4 will tend to rise, can that
be changed to a tendency to fall by adding NO2? Similarly, if you said the
pressure of N2O4 will tend to fall, can that be changed to a tendency to
rise by adding NO2?
If you said the tendency can be reversed in the second question, calculate
the minimum pressure of NO 2 needed to reverse it.
Round your answer to 2 significant digits.
yes
no
0.42 atm
☑
5
0/5
?
مله
Ar
Chapter 29 Solutions
PKG ORGANIC CHEMISTRY
Ch. 29 - Prob. 29.1PCh. 29 - Problem 29.2
What form exists at the isoelectric...Ch. 29 - Problem 29.3
Explain why the of the group of an...Ch. 29 - Prob. 29.4PCh. 29 - Problem 29.5
What -halo carbonyl compound is...Ch. 29 - Problem 29.6
The enolate derived from diethyl...Ch. 29 - Problem 29.7
What amino acid is formed when is...Ch. 29 - Problem 29.8
What aldehyde is needed to synthesize...Ch. 29 - Problem 29.9
Draw the products of each...Ch. 29 - Prob. 29.10P
Ch. 29 - Prob. 29.11PCh. 29 - Prob. 29.12PCh. 29 - Problem 29.13
What alkene is needed to synthesize...Ch. 29 - Problem 29.14
Draw the structure of each peptide....Ch. 29 - Problem 29.15
Name each peptide using both the...Ch. 29 - Prob. 29.16PCh. 29 - Prob. 29.17PCh. 29 - Problem 29.18
Glutathione, a powerful antioxidant...Ch. 29 - Problem 29.19
Draw the structure of the...Ch. 29 - Problem 29.20
Give the amino acid sequence of an...Ch. 29 - a What products are formed when each peptide is...Ch. 29 - Prob. 29.22PCh. 29 - Devise a synthesis of each peptide from amino acid...Ch. 29 - Devise a synthesis of the following dipeptide from...Ch. 29 - Prob. 29.25PCh. 29 - Consider two molecules of a tetrapeptide composed...Ch. 29 - What types of stabilizing interactions exist...Ch. 29 - Prob. 29.28PCh. 29 - Draw the product formed when the following amino...Ch. 29 - With reference to the following peptide: a...Ch. 29 - Devise a synthesis of the following dipeptide from...Ch. 29 - Prob. 29.32PCh. 29 - Histidine is classified as a basic amino acid...Ch. 29 - Tryptophan is not classified as a basic amino acid...Ch. 29 - What is the structure of each amino acid at its...Ch. 29 - What is the predominant form of each of the...Ch. 29 - 29.37 What is the predominant form of each of the...Ch. 29 - a. Draw the structure of the tripeptide A–A–A, and...Ch. 29 - 29.39 Draw the organic products formed in each...Ch. 29 - 29.40 What alkyl halide is needed to synthesize...Ch. 29 - 29.41 Devise a synthesis of threonine from diethyl...Ch. 29 - 29.42 Devise a synthesis of each amino acid from...Ch. 29 - Prob. 29.43PCh. 29 - Prob. 29.44PCh. 29 - Prob. 29.45PCh. 29 - Prob. 29.46PCh. 29 - Prob. 29.47PCh. 29 - 29.48 Brucine is a poisonous alkaloid obtained...Ch. 29 - Prob. 29.49PCh. 29 - Prob. 29.50PCh. 29 - Draw the structure for each peptide: (a) Phe–Ala;...Ch. 29 - 29.52 For the tetrapeptide Asp–Arg–Val–Tyr:
a....Ch. 29 - Prob. 29.53PCh. 29 - Prob. 29.54PCh. 29 - 29.55 Draw the amino acids and peptide fragments...Ch. 29 - Prob. 29.56PCh. 29 - Prob. 29.57PCh. 29 - Prob. 29.58PCh. 29 - 29.59 An octapeptide contains the following amino...Ch. 29 - 29.60 Draw the organic products formed in each...Ch. 29 - Draw all the steps in the synthesis of each...Ch. 29 - 29.62 Write out the steps for the synthesis of...Ch. 29 - 29.63 Besides the Boc and Fmoc protecting groups...Ch. 29 - 29.64 Another method to form a peptide bond...Ch. 29 - 29.65 Draw the mechanism for the reaction that...Ch. 29 - 29.66 Which of the following amino acids are...Ch. 29 - 29.67 After the peptide chain of collagen has been...Ch. 29 - Prob. 29.68PCh. 29 - Prob. 29.69PCh. 29 - 29.70 The anti-obesity drug orlistat works by...Ch. 29 - Prob. 29.71P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Homework 13 (Ch17) Question 4 of 4 (1 point) | Question Attempt: 2 of 2 ✓ 1 ✓ 2 = 3 4 Time Remaining: 4:25:54 Using the thermodynamic information in the ALEKS Data tab, calculate the standard reaction free energy of the following chemical reaction: 2CH3OH (g)+302 (g) → 2CO2 (g) + 4H₂O (g) Round your answer to zero decimal places. ☐ kJ x10 ☐ Subm Check 2020 Hill LLC. All Rights Reserved. Terms of Use | Privacy Cearrow_forwardIdentifying the major species in weak acid or weak base equilibria Your answer is incorrect. • Row 2: Your answer is incorrect. • Row 3: Your answer is incorrect. • Row 6: Your answer is incorrect. 0/5 The preparations of two aqueous solutions are described in the table below. For each solution, write the chemical formulas of the major species present at equilibrium. You can leave out water itself. Write the chemical formulas of the species that will act as acids in the 'acids' row, the formulas of the species that will act as bases in the 'bases' row, and the formulas of the species that will act as neither acids nor bases in the 'other' row. You will find it useful to keep in mind that HF is a weak acid. acids: HF 0.1 mol of NaOH is added to 1.0 L of a 0.7M HF solution. bases: 0.13 mol of HCl is added to 1.0 L of a solution that is 1.0M in both HF and KF. Exponent other: F acids: HF bases: F other: K 1 0,0,... ? 000 18 Ararrow_forwardUsing reaction free energy to predict equilibrium composition Consider the following equilibrium: 2NOCI (g) 2NO (g) + Cl2 (g) AGº =41. kJ Now suppose a reaction vessel is filled with 4.50 atm of nitrosyl chloride (NOCI) and 6.38 atm of chlorine (C12) at 212. °C. Answer the following questions about this system: ? rise Under these conditions, will the pressure of NOCI tend to rise or fall? x10 fall Is it possible to reverse this tendency by adding NO? In other words, if you said the pressure of NOCI will tend to rise, can that be changed to a tendency to fall by adding NO? Similarly, if you said the pressure of NOCI will tend to fall, can that be changed to a tendency to rise by adding NO? yes no If you said the tendency can be reversed in the second question, calculate the minimum pressure of NO needed to reverse it. Round your answer to 2 significant digits. 0.035 atm ✓ G 00. 18 Ararrow_forward
- Highlight each glycosidic bond in the molecule below. Then answer the questions in the table under the drawing area. HO- HO- -0 OH OH HO NG HO- HO- OH OH OH OH NG OHarrow_forward€ + Suppose the molecule in the drawing area below were reacted with H₂ over a platinum catalyst. Edit the molecule to show what would happen to it. That is, turn it into the product of the reaction. Also, write the name of the product molecule under the drawing area. Name: ☐ H C=0 X H- OH HO- H HO- -H CH₂OH ×arrow_forwardDraw the Haworth projection of the disaccharide made by joining D-glucose and D-mannose with a ẞ(1-4) glycosidic bond. If the disaccharide has more than one anomer, you can draw any of them. Click and drag to start drawing a structure. Xarrow_forward
- Epoxides can be opened in aqueous acid or aqueous base to produce diols (molecules with two OH groups). In this question, you'll explore the mechanism of epoxide opening in aqueous acid. 2nd attempt Be sure to show all four bonds at stereocenters using hash and wedge lines. 0 0 Draw curved arrows to show how the epoxide reacts with hydronium ion. 100 +1: 1st attempt Feedback Be sure to show all four bonds at stereocenters using hash and wedge lines. See Periodic Table See Hint H A 5 F F Hr See Periodic Table See Hintarrow_forward03 Question (1 point) For the reaction below, draw both of the major organic products. Be sure to consider stereochemistry. > 1. CH₂CH₂MgBr 2. H₂O 3rd attempt Draw all four bonds at chiral centers. Draw all stereoisomers formed. Draw the structures here. e 130 AN H See Periodic Table See Hint P C Brarrow_forwardYou may wish to address the following issues in your response if they are pertinent to the reaction(s) you propose to employ:1) Chemoselectivity (why this functional group and not another?) 2) Regioselectivity (why here and not there?) 3) Stereoselectivity (why this stereoisomer?) 4) Changes in oxidation state. Please make it in detail and draw it out too in what step what happens. Thank you for helping me!arrow_forward
- 1) Chemoselectivity (why this functional group and not another?) 2) Regioselectivity (why here and not there?) 3) Stereoselectivity (why this stereoisomer?) 4) Changes in oxidation state. Everything in detail and draw out and write it.arrow_forwardCalculating the pH at equivalence of a titration 3/5 Izabella A chemist titrates 120.0 mL of a 0.7191M dimethylamine ((CH3)2NH) solution with 0.5501 M HBr solution at 25 °C. Calculate the pH at equivalence. The pk of dimethylamine is 3.27. Round your answer to 2 decimal places. Note for advanced students: you may assume the total volume of the solution equals the initial volume plus the volume of HBr solution added. pH = ☐ ✓ 18 Ar Boarrow_forwardAlcohols can be synthesized using an acid-catalyzed hydration of an alkene. An alkene is combined with aqueous acid (e.. sulfuric acid in water). The reaction mechanism typically involves a carbocation intermediate. > 3rd attempt 3343 10 8 Draw arrows to show the reaction between the alkene and hydronium ion. that 2nd attempt Feedback 1st attempt تعمال Ju See Periodic Table See Hint F D Ju See Periodic Table See Hintarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div
World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

World of Chemistry
Chemistry
ISBN:9780618562763
Author:Steven S. Zumdahl
Publisher:Houghton Mifflin College Div
DIGESTER-35 | VITAMINS AND THEIR RELATED COENZYMES| GPAT | NIPER | PHARMACIST| DI; Author: GPAT DISCUSSION CENTER;https://www.youtube.com/watch?v=CGrdNYmho0s;License: Standard YouTube License, CC-BY