
Concept explainers
Interpretation:
[1,3]sigmatropic migrations of hydrogen cannot occur under thermal conditions but [1,3] sigmatropic migration of carbon can. Reason for this should be explained.
Concept introduction:
Pericyclic reactions are “ any concerted reaction in which bonds are formed or brocken in a cyclic transition state”. There is a single transition state from start to finish, in contrast to a stepwise reaction.
There are mainly three types of pericyclic reactions,
- 1) Electrocyclic reactions
- 2) Cycloaddition reactions
- 3) Sigmatropic reactions
In a sigmatropic reaction “ one new sigma-bond is formed as another breaks.”
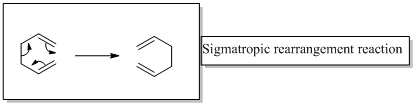
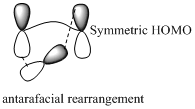
Sigmatropic rearrangement reactions are named with digits. For example a [1, 3] sigmatropic rearrangement describe a reaction in which the residue migrates from position 1 to position 3. Migration of carbon and hydrogen will occur in a sigmatropic rearrangement reaction. When hydrogen migrates in a sigmatropic rearrangement, the s orbital of the hydrogen is partially bonded to both the migration origin and the migration terminus in the transition state. Migration of hydrogen in suprafacial and antarafacial rearrangement can be represented as follows,


Migration of carbon occurs through two ways because it has a two lobed p orbital. Carbon can simultaneously interact with the migration origin and the migration terminus using one lobe of its p orbital.
Migration of carbon in suprafacial and antarafacial rearrangement can be represented as follows,
Carbon migrating with one lobe of its p orbital interacting


Carbon migrating with both lobe of its p orbital interacting

Want to see the full answer?
Check out a sample textbook solution
Chapter 28 Solutions
Organic Chemistry; Modified MasteringChemistry with Pearson eText -- ValuePack Access Card; Study Guide and Student Solutions Manual for Organic Chemistry, Books a la Carte Edition (7th Edition)
- -C = C - C - + Br₂ + I" -> -C-C-c -C = C -C- + Br² + I₂ -C=C Br I + Brū + Iz -7- C - C-C- I Br Mechanism; - C = c - c - + Br - Br > - C-c-c- Br -C-C-C- + 1 - - -Ċ-Ċ'-c' - Br Br Iarrow_forwardWrite the mechanism of the esterification reaction (please show the mechanism included line pairs and arrows)arrow_forwardHow do I break down the reaction shown on the chalkboard and explain it correctly using the bromonium ion mechanism, instead of the (disproven) carbocation-based mechanismarrow_forward
- ¿Qué the product is obtained from tetraethoxypropano and hidrazina?. Indicate the reason why the corresponding dial is used.arrow_forwardIf CH3COCH2CH(OCH3)2 is reacted with hydrazine, two isomeric products are formed. Indicate their structures and the major product.arrow_forwardIs it possible to obtain addition derivatives to nitrogen in position 2 of pyrazoles by reaction with electrophilic agents? Reason for this.arrow_forward
- Starting from 1,3-dicarbonyl derivatives to obtain isooxazoles and isothiazoles. Indicate whether synthetic methods exist.arrow_forwardIn the synthesis of benzotriazole, adding NaNO2 heats the solution. State the reason.arrow_forwardIndicate the products obtained by treating benzotriazole with dimethyl sulfate or methyl iodide in a basic medium.arrow_forward
 Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,
Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,


