
Concept explainers
Interpretation:
It should be determined that the given [1, 7] sigmatropic rearrangement involves whether suprafacial or antarafacial rearrangement.
Concept introduction:
In a sigmatropic reaction “ one new sigma-bond is formed as another breaks.”
Sigmatropic rearrangement reactions are named with digits. For example a [1, 3] sigmatropic rearrangement describe a reaction in which the residue migrates from position 1 to position 3.
Migration of carbon and hydrogen will occur in a sigmatropic rearrangement reaction.
When hydrogen migrates in a sigmatropic rearrangement, the s orbital of the hydrogen is partially bonded to both the migration origin and the migration terminus in the transition state.
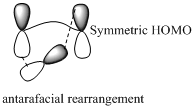
Migration of hydrogen in suprafacial and antarafacial rearrangement can be represented as follows,


Migration of carbon occurs through two ways because it has a two lobed p orbital. Carbon can simultaneously interact with the migration origin and the migration terminus using one lobe of its p orbital. Migration of carbon in suprafacial and antarafacial rearrangement can be represented as follows,
Carbon migrating with one lobe of its p orbital interacting


Carbon migrating with both lobe of its p orbital interacting

Trending nowThis is a popular solution!

Chapter 28 Solutions
Organic Chemistry; Modified MasteringChemistry with Pearson eText -- ValuePack Access Card; Study Guide and Student Solutions Manual for Organic Chemistry, Books a la Carte Edition (7th Edition)
- i need help identifying the four carbon oxygen bonds in the following:arrow_forwardImagine each of the molecules shown below was found in an aqueous solution. Can you tell whether the solution is acidic, basic, or neutral? molecule HO H3N + The solution is... X O acidic OH O basic H3N-CH-C-O O neutral ○ (unknown) O acidic ○ basic CH2 CH 3-S-CH2 O neutral ○ (unknown) H3N O OH O acidic O basic Oneutral O (unknown) 0 H3N-CH-C-O CH3 CH CH3 O acidic O basic O neutral ○ (unknown) ? olo Ar BHarrow_forwardno Ai walkthroughs need other product (product in picture is wrong dont submit the same thing)arrow_forward
- I have a 2 mil plastic film that degrades after 22 days at 88C and at 61C takes 153 days. What is the failure at 47C in days.arrow_forwardIf a 5 film plastic film degraded in 30 days at 35C and the same film degraded in 10 days at 55 C and 2 days at 65C what would the predicted life time be at 22C for the same film?arrow_forwardno Ai walkthroughsarrow_forward
- I have a aqueous solution (175 ml) of iridium trichloride containing 8,750 ppm Iridium by ICP OES analysis. What is the percent concentration of Iridium trichloride in aquous solution and provide the concentration in moles per liter, percentage by weight.arrow_forwardno Ai walkthroughsarrow_forwardno Ai walkthroughsarrow_forward

 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


