
THERMODYNAMICS (LL)-W/ACCESS >CUSTOM<
9th Edition
ISBN: 9781266657610
Author: CENGEL
Publisher: MCG CUSTOM
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 2.8, Problem 40P
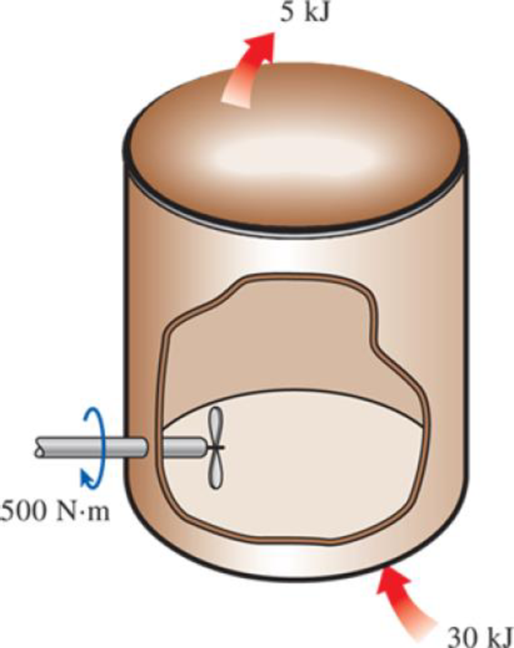
Water is being heated in a closed pan on top of a range while being stirred by a paddle wheel. During the process, 30 kJ of heat is transferred to the water, and 5 kJ of heat is lost to the surrounding air. The paddle-wheel work amounts to 500 N·m. Determine the final energy of the system if its initial energy is 12.5 kJ.
FIGURE P2–40
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
The answers to this question s wasn't properly given, I need expert handwritten solutions
I need expert handwritten solutions to this only
Two large tanks, each holding 100 L of liquid, are interconnected by pipes, with the liquid flowing from tank
A into tank B at a rate of 3 L/min and from B into A at a rate of 1 L/min (see Figure Q1). The liquid inside each
tank is kept well stirred. A brine solution with a concentration of 0.2 kg/L of salt flows into tank A at a rate of
6 L/min. The diluted solution flows out of the system from tank A at 4 L/min and from tank B at 2 L/min. If,
initially, tank A contains pure water and tank B contains 20 kg of salt.
A
6 L/min
0.2 kg/L
x(t)
100 L
4 L/min
x(0) = 0 kg
3 L/min
B
y(t)
100 L
y(0) = 20 kg
2 L/min
1 L/min
Figure Q1 - Mixing problem for interconnected tanks
Determine the mass of salt in each tank at time t > 0:
Analytically (hand calculations)
Chapter 2 Solutions
THERMODYNAMICS (LL)-W/ACCESS >CUSTOM<
Ch. 2.8 - What is the difference between the macroscopic and...Ch. 2.8 - What is total energy? Identify the different forms...Ch. 2.8 - List the forms of energy that contribute to the...Ch. 2.8 - How are heat, internal energy, and thermal energy...Ch. 2.8 - What is mechanical energy? How does it differ from...Ch. 2.8 - Portable electric heaters are commonly used to...Ch. 2.8 - Natural gas, which is mostly methane CH4, is a...Ch. 2.8 - Consider the falling of a rock off a cliff into...Ch. 2.8 - Electric power is to be generated by installing a...Ch. 2.8 - The specific kinetic energy of a moving mass is...
Ch. 2.8 - Determine the specific kinetic energy of a mass...Ch. 2.8 - Calculate the total potential energy, in Btu, of...Ch. 2.8 - Determine the specific potential energy, in kJ/kg,...Ch. 2.8 - An object whose mass is 100 kg is located 20 m...Ch. 2.8 - A water jet that leaves a nozzle at 60 m/s at a...Ch. 2.8 - Consider a river flowing toward a lake at an...Ch. 2.8 - At a certain location, wind is blowing steadily at...Ch. 2.8 - What is the caloric theory? When and why was it...Ch. 2.8 - In what forms can energy cross the boundaries of a...Ch. 2.8 - What is an adiabatic process? What is an adiabatic...Ch. 2.8 - When is the energy crossing the boundaries of a...Ch. 2.8 - Consider an automobile traveling at a constant...Ch. 2.8 - A room is heated by an iron that is left plugged...Ch. 2.8 - A room is heated as a result of solar radiation...Ch. 2.8 - A gas in a pistoncylinder device is compressed,...Ch. 2.8 - A small electrical motor produces 5 W of...Ch. 2.8 - A car is accelerated from rest to 85 km/h in 10 s....Ch. 2.8 - A construction crane lifts a prestressed concrete...Ch. 2.8 - Determine the torque applied to the shaft of a car...Ch. 2.8 - A spring whose spring constant is 200 lbf/in has...Ch. 2.8 - How much work, in kJ, can a spring whose spring...Ch. 2.8 - A ski lift has a one-way length of 1 km and a...Ch. 2.8 - The engine of a 1500-kg automobile has a power...Ch. 2.8 - A damaged 1200-kg car is being towed by a truck....Ch. 2.8 - As a spherical ammonia vapor bubble rises in...Ch. 2.8 - A steel rod of 0.5 cm diameter and 10 m length is...Ch. 2.8 - What are the different mechanisms for transferring...Ch. 2.8 - For a cycle, is the net work necessarily zero? For...Ch. 2.8 - On a hot summer day, a student turns his fan on...Ch. 2.8 - Water is being heated in a closed pan on top of a...Ch. 2.8 - An adiabatic closed system is accelerated from 0...Ch. 2.8 - A fan is to accelerate quiescent air to a velocity...Ch. 2.8 - A vertical pistoncylinder device contains water...Ch. 2.8 - At winter design conditions, a house is projected...Ch. 2.8 - A water pump increases the water pressure from 15...Ch. 2.8 - The lighting needs of a storage room are being met...Ch. 2.8 - A university campus has 200 classrooms and 400...Ch. 2.8 - Consider a room that is initially at the outdoor...Ch. 2.8 - An escalator in a shopping center is designed to...Ch. 2.8 - Consider a 2100-kg car cruising at constant speed...Ch. 2.8 - Prob. 51PCh. 2.8 - What is mechanical efficiency? What does a...Ch. 2.8 - How is the combined pumpmotor efficiency of a pump...Ch. 2.8 - Can the combined turbinegenerator efficiency be...Ch. 2.8 - Consider a 2.4-kW hooded electric open burner in...Ch. 2.8 - The steam requirements of a manufacturing facility...Ch. 2.8 - Reconsider Prob. 256E. Using appropriate software,...Ch. 2.8 - A 75-hp (shaft output) motor that has an...Ch. 2.8 - Prob. 59PCh. 2.8 - An exercise room has six weight-lifting machines...Ch. 2.8 - A room is cooled by circulating chilled water...Ch. 2.8 - The water in a large lake is to be used to...Ch. 2.8 - A 7-hp (shaft) pump is used to raise water to an...Ch. 2.8 - A geothermal pump is used to pump brine whose...Ch. 2.8 - At a certain location, wind is blowing steadily at...Ch. 2.8 - Reconsider Prob. 265. Using appropriate software,...Ch. 2.8 - Water is pumped from a lower reservoir to a higher...Ch. 2.8 - An 80-percent-efficient pump with a power input of...Ch. 2.8 - Water is pumped from a lake to a storage tank 15 m...Ch. 2.8 - Large wind turbines with a power capacity of 8 MW...Ch. 2.8 - A hydraulic turbine has 85 m of elevation...Ch. 2.8 - The water behind Hoover Dam in Nevada is 206 m...Ch. 2.8 - An oil pump is drawing 44 kW of electric power...Ch. 2.8 - A wind turbine is rotating at 15 rpm under steady...Ch. 2.8 - How does energy conversion affect the environment?...Ch. 2.8 - What is acid rain? Why is it called a rain? How do...Ch. 2.8 - Why is carbon monoxide a dangerous air pollutant?...Ch. 2.8 - What is the greenhouse effect? How does the excess...Ch. 2.8 - What is smog? What does it consist of? How does...Ch. 2.8 - Consider a household that uses 14,000 kWh of...Ch. 2.8 - When a hydrocarbon fuel is burned, almost all of...Ch. 2.8 - Prob. 82PCh. 2.8 - A typical car driven 20,000 km a year emits to the...Ch. 2.8 - Prob. 84PCh. 2.8 - What are the mechanisms of heat transfer?Ch. 2.8 - Which is a better heat conductor, diamond or...Ch. 2.8 - How does forced convection differ from natural...Ch. 2.8 - What is a blackbody? How do real bodies differ...Ch. 2.8 - Define emissivity and absorptivity. What is...Ch. 2.8 - Does any of the energy of the sun reach the earth...Ch. 2.8 - The inner and outer surfaces of a 5-m 6-m brick...Ch. 2.8 - The inner and outer surfaces of a 0.5-cm-thick 2-m...Ch. 2.8 - Reconsider Prob. 292. Using appropriate software,...Ch. 2.8 - Prob. 94PCh. 2.8 - Prob. 95PCh. 2.8 - Prob. 96PCh. 2.8 - Prob. 97PCh. 2.8 - For heat transfer purposes, a standing man can be...Ch. 2.8 - Prob. 99PCh. 2.8 - Prob. 100PCh. 2.8 - A 1000-W iron is left on the ironing board with...Ch. 2.8 - A 7-cm-external-diameter, 18-m-long hot-water pipe...Ch. 2.8 - A thin metal plate is insulated on the back and...Ch. 2.8 - Reconsider Prob. 2103. Using appropriate software,...Ch. 2.8 - The outer surface of a spacecraft in space has an...Ch. 2.8 - Prob. 106PCh. 2.8 - A hollow spherical iron container whose outer...Ch. 2.8 - Some engineers have developed a device that...Ch. 2.8 - Consider a classroom for 55 students and one...Ch. 2.8 - Consider a homeowner who is replacing his...Ch. 2.8 - Prob. 111RPCh. 2.8 - The U.S. Department of Energy estimates that...Ch. 2.8 - A typical household pays about 1200 a year on...Ch. 2.8 - Prob. 114RPCh. 2.8 - Prob. 115RPCh. 2.8 - Prob. 116RPCh. 2.8 - Consider a TV set that consumes 120 W of electric...Ch. 2.8 - Water is pumped from a 200-ft-deep well into a...Ch. 2.8 - Consider a vertical elevator whose cabin has a...Ch. 2.8 - Prob. 120RPCh. 2.8 - In a hydroelectric power plant, 65 m3/s of water...Ch. 2.8 - The demand for electric power is usually much...Ch. 2.8 - The pump of a water distribution system is powered...Ch. 2.8 - Prob. 124RPCh. 2.8 - A 2-kW electric resistance heater in a room is...Ch. 2.8 - Prob. 126FEPCh. 2.8 - A 75-hp compressor in a facility that operates at...Ch. 2.8 - On a hot summer day, the air in a well-sealed room...Ch. 2.8 - A fan is to accelerate quiescent air to a velocity...Ch. 2.8 - A 900-kg car cruising at a constant speed of 60...Ch. 2.8 - Prob. 131FEPCh. 2.8 - Prob. 132FEPCh. 2.8 - A 2-kW pump is used to pump kerosene ( = 0.820...Ch. 2.8 - Prob. 134FEPCh. 2.8 - Prob. 135FEPCh. 2.8 - Prob. 136FEPCh. 2.8 - Prob. 137FEPCh. 2.8 - Heat is transferred steadily through a...Ch. 2.8 - The roof of an electrically heated house is 7 m...
Additional Engineering Textbook Solutions
Find more solutions based on key concepts
What is an uninitialized variable?
Starting Out with Programming Logic and Design (5th Edition) (What's New in Computer Science)
CONCEPT QUESTIONS
15.CQ3 The ball rolls without slipping on the fixed surface as shown. What is the direction ...
Vector Mechanics for Engineers: Statics and Dynamics
Why is the study of database technology important?
Database Concepts (8th Edition)
Assume a telephone signal travels through a cable at two-thirds the speed of light. How long does it take the s...
Electric Circuits. (11th Edition)
How is the hydrodynamic entry length defined for flow in a pipe? Is the entry length longer in laminar or turbu...
Fluid Mechanics: Fundamentals and Applications
17–1C A high-speed aircraft is cruising in still air. How does the temperature of air at the nose of the aircra...
Thermodynamics: An Engineering Approach
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Similar questions
- Two springs and two masses are attached in a straight vertical line as shown in Figure Q3. The system is set in motion by holding the mass m₂ at its equilibrium position and pushing the mass m₁ downwards of its equilibrium position a distance 2 m and then releasing both masses. if m₁ = m₂ = 1 kg, k₁ = 3 N/m and k₂ = 2 N/m. www.m k₁ = 3 (y₁ = 0). m₁ = 1 k2=2 (y₂ = 0) |m₂ = 1 Y2 y 2 System in static equilibrium (Net change in spring length =32-31) System in motion Figure Q3 - Coupled mass-spring system Determine the equations of motion y₁(t) and y₂(t) for the two masses m₁ and m₂ respectively: Analytically (hand calculations)arrow_forward100 As a spring is heated, its spring constant decreases. Suppose the spring is heated and then cooled so that the spring constant at time t is k(t) = t sin N/m. If the mass-spring system has mass m = 2 kg and a damping constant b = 1 N-sec/m with initial conditions x(0) = 6 m and x'(0) = -5 m/sec and it is subjected to the harmonic external force f(t) = 100 cos 3t N. Find at least the first four nonzero terms in a power series expansion about t = 0, i.e. Maclaurin series expansion, for the displacement: Analytically (hand calculations)arrow_forwardthis is answer to a vibrations question. in the last part it states an assumption of x2, im not sure where this assumption comes from. an answer would be greatly appreciatedarrow_forward
- Please answer with the sketches.arrow_forwardThe beam is made of elastic perfectly plastic material. Determine the shape factor for the cross section of the beam (Figure Q3). [Take σy = 250 MPa, yNA = 110.94 mm, I = 78.08 x 106 mm²] y 25 mm 75 mm I 25 mm 200 mm 25 mm 125 Figure Q3arrow_forwardA beam of the cross section shown in Figure Q3 is made of a steel that is assumed to be elastic- perfectectly plastic material with E = 200 GPa and σy = 240 MPa. Determine: i. The shape factor of the cross section ii. The bending moment at which the plastic zones at the top and bottom of the bar are 30 mm thick. 15 mm 30 mm 15 mm 30 mm 30 mm 30 mmarrow_forward
- A torque of magnitude T = 12 kNm is applied to the end of a tank containing compressed air under a pressure of 8 MPa (Figure Q1). The tank has a 180 mm inner diameter and a 12 mm wall thickness. As a result of several tensile tests, it has been found that tensile yeild strength is σy = 250 MPa for thr grade of steel used. Determine the factor of safety with respect to yeild, using: (a) The maximum shearing stress theory (b) The maximum distortion energy theory T Figure Q1arrow_forwardAn external pressure of 12 MPa is applied to a closed-end thick cylinder of internal diameter 150 mm and external diameter 300 mm. If the maximum hoop stress on the inner surface of the cylinder is limited to 30 MPa: (a) What maximum internal pressure can be applied to the cylinder? (b) Sketch the variation of hoop and radial stresses across the cylinder wall. (c) What will be the change in the outside diameter when the above pressure is applied? [Take E = 207 GPa and v = 0.29]arrow_forwardso A 4 I need a detailed drawing with explanation し i need drawing in solution motion is as follows; 1- Dwell 45°. Plot the displacement diagram for a cam with flat follower of width 14 mm. The required 2- Rising 60 mm in 90° with Simple Harmonic Motion. 3- Dwell 90°. 4- Falling 60 mm for 90° with Simple Harmonic Motion. 5- Dwell 45°. cam is 50 mm. Then design the cam profile to give the above displacement diagram if the minimum circle diameter of the か ---2-125 750 x2.01 98Parrow_forward
- Figure below shows a link mechanism in which the link OA rotates uniformly in an anticlockwise direction at 10 rad/s. the lengths of the various links are OA=75 mm, OB-150 mm, BC=150 mm, CD-300 mm. Determine for the position shown, the sliding velocity of D. A 45 B Space Diagram o NTS (Not-to-Scale) C Darrow_forwardI need a detailed drawing with explanation so Solle 4 يكا Pax Pu + 96** motion is as follows; 1- Dwell 45°. Plot the displacement diagram for a cam with flat follower of width 14 mm. The required 2- Rising 60 mm in 90° with Simple Harmonic Motion. 3- Dwell 90°. 4- Falling 60 mm for 90° with Simple Harmonic Motion. 5- Dwell 45°. cam is 50 mm. Then design the cam profile to give the above displacement diagram if the minimum circle diameter of the 55 ---20125 750 X 2.01 1989arrow_forwardAshaft fitted with a flywheel rotates at 300 rpm. and drives a machine. The torque required to drive the machine varies in a cyclic manner over a period of 2 revolutions. The torque drops from 20,000 Nm to 10,000 Nm uniformly during 90 degrees and remains constant for the following 180 degrees. It then rises uniformly to 35,000 Nm during the next 225 degrees and after that it drops to 20,000 in a uniform manner for 225 degrees, the cycle being repeated thereafter. Determine the power required to drive the machine and percentage fluctuation in speed, if the driving torque applied to the shaft is constant and the mass of the flywheel is 12 tonnes with radius of gyration of 500 mm. What is the maximum angular acceleration of the flywheel. 35,000 TNM 20,000 10,000 0 90 270 495 Crank angle 8 degrees 720arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY

Elements Of Electromagnetics
Mechanical Engineering
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:9780134319650
Author:Russell C. Hibbeler
Publisher:PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:9781259822674
Author:Yunus A. Cengel Dr., Michael A. Boles
Publisher:McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:9781118170519
Author:Norman S. Nise
Publisher:WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:9781337093347
Author:Barry J. Goodno, James M. Gere
Publisher:Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:9781118807330
Author:James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:WILEY
First Law of Thermodynamics, Basic Introduction - Internal Energy, Heat and Work - Chemistry; Author: The Organic Chemistry Tutor;https://www.youtube.com/watch?v=NyOYW07-L5g;License: Standard youtube license