
Concept explainers
(a)
Interpretation: The relationship between ![]() and
and ![]() is to be stated.
is to be stated.
Concept introduction: The anomers are cyclic monosaccharides, which differ in configuration at one stereogenic centre. These carbon atoms are called anomeric centre.
Answer to Problem 28.42P
The compounds ![]() and
and ![]() are epimers.
are epimers.
Explanation of Solution
The structures of compounds ![]() and
and ![]() are,
are,
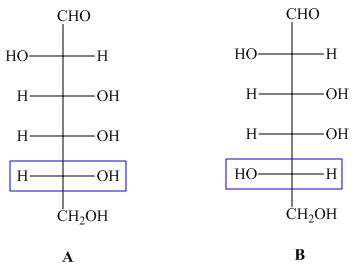
Figure 1
In the given structures, the configuration at ![]() is different. Hence, the compounds
is different. Hence, the compounds ![]() and
and ![]() are epimers.
are epimers.
The compounds ![]() and
and ![]() are epimers.
are epimers.
(b)
Interpretation: The relationship between ![]() and
and ![]() is to be stated.
is to be stated.
Concept introduction: The anomers are cyclic monosaccharides, which differ in configuration at one stereogenic centre. These carbon atoms are called anomeric centre. The compounds which are neither mirror images nor supperimposable on each other are known as diastereomer.
Answer to Problem 28.42P
The compound ![]() and
and ![]() are diastreomers but not epimers.
are diastreomers but not epimers.
Explanation of Solution
The structures of given compound ![]() and
and ![]() are,
are,
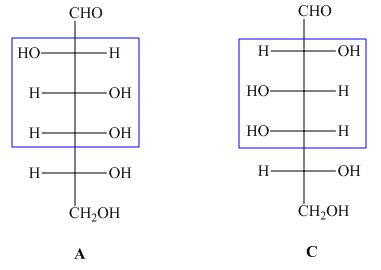
Figure 2
In the given structures, the compound ![]() is not mirror image of compound
is not mirror image of compound ![]() and the configuration of more than is different. Hence, the compound
and the configuration of more than is different. Hence, the compound ![]() and
and ![]() are diastreomers but not epimers.
are diastreomers but not epimers.
The compound ![]() and
and ![]() are diastreomers but not epimers.
are diastreomers but not epimers.
(c)
Interpretation: The relationship between ![]() and
and ![]() is to be stated.
is to be stated.
Concept introduction: The compounds which are mirror images as well as non-supperimposable on each other. These compounds are known as enantiomers.
Answer to Problem 28.42P
The compound ![]() and
and ![]() are enantiomers.
are enantiomers.
Explanation of Solution
The structures of given compound ![]() and
and ![]() are,
are,
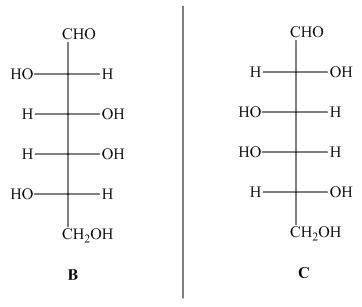
Figure 3
In the given structures, the compound ![]() is mirror image of compound
is mirror image of compound ![]() . Hence, the compound
. Hence, the compound ![]() and
and ![]() are enantiomers.
are enantiomers.
The compound ![]() and
and ![]() are enantiomers.
are enantiomers.
(d)
Interpretation: The relationship between ![]() and
and ![]() is to be stated.
is to be stated.
Concept introduction: The compounds which have same molecular formula but differ in connectivity of the substituents. These compounds are known as constitutional isomers.
Answer to Problem 28.42P
The compound ![]() and
and ![]() are constitutional isomers.
are constitutional isomers.
Explanation of Solution
The structures of given compound ![]() and
and ![]() are,
are,
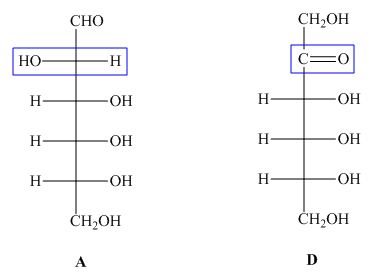
Figure 4
In the given structures, the molecular formula of ![]() is same to the molecular formula of
is same to the molecular formula of ![]() but the connectivity of substituents at
but the connectivity of substituents at ![]() is different. Hence, the compound
is different. Hence, the compound ![]() and
and ![]() are constitutional isomers.
are constitutional isomers.
The compound ![]() and
and ![]() are constitutional isomers.
are constitutional isomers.
(e)
Interpretation: The relationship between ![]() and
and ![]() is to be stated.
is to be stated.
Concept introduction: The anomers are cyclic monosaccharides, which differ in configuration at one stereogenic centre. These carbon atoms are called anomeric centre. The compounds which are neither mirror images nor supperimposable on each other are known as diastereomer.
Answer to Problem 28.42P
The compounds ![]() and
and ![]() are diastreomers but not epimers.
are diastreomers but not epimers.
Explanation of Solution
The substituents on a carbon are present above the ring in Haworth projection indicates that these bonds are above the plane (i.e. either equatorial or axial position) in chair form. The structures of given compound ![]() and
and ![]() are,
are,
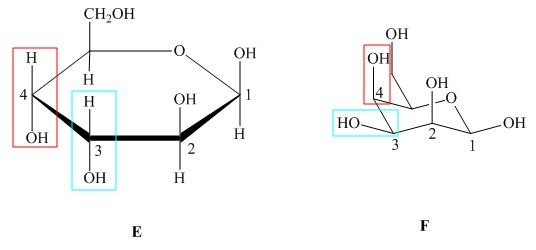
Figure 5
In the given structures, the configuration at ![]() is different which shows that the compound
is different which shows that the compound ![]() is not mirror image of compound
is not mirror image of compound ![]() and. Hence, the compound
and. Hence, the compound ![]() and
and ![]() are diastreomers but not epimers.
are diastreomers but not epimers.
The compounds ![]() and
and ![]() are diastreomers but not epimers.
are diastreomers but not epimers.
Want to see more full solutions like this?
Chapter 28 Solutions
Package: Loose Leaf for Organic Chemistry with Biological Topics with Connect Access Card
- Draw the major product of this reaction. Ignore inorganic byproducts. Assume that the water side product is continuously removed to drive the reaction toward products. O CH3CH2NH2, TSOH Select to Draw >arrow_forwardPredict the major organic product(s) for the following reaction.arrow_forwardPredict the major organic product(s) for the following reactions.arrow_forward
- Provide the complete mechanism for the reactions below. You must include appropriate arrows,intermediates, and formal charges.arrow_forwardIndicate the products obtained by reacting fluorobenzene with a sulfonitric mixture.arrow_forwardIf I have 1-bromopropene, to obtain compound A, I have to add NaOH and another compound. Indicate which compound that would be. C6H5 CH3arrow_forward
- If I have 1-bromopropene and I want to obtain (1,1-dipropoxyethyl)benzene, indicate the compound that I should add in addition to NaOH.arrow_forwardDraw the major product of this reaction. Ignore inorganic byproducts. Ο HSCH2CH2CH2SH, BF3 Select to Draw I Submitarrow_forwardFeedback (7/10) Draw the major product of this reaction. Ignore inorganic byproducts. Assume that the water side product is continuously removed to drive the reaction toward products. Incorrect, 3 attempts remaining Ο (CH3CH2)2NH, TSOH Select to Draw V N. 87% Retryarrow_forward
- If I want to obtain (1,1-dipropoxyethyl)benzene from 1-bromopropene, indicate the product that I have to add in addition to NaOH.arrow_forwardIndicate the products obtained when fluorobenzene reacts with a sulfonitric acid mixture (HNO3 + H2SO4). Indicate the majority if necessary.arrow_forwardIndicate the products obtained when chlorobenzene acid reacts with a sulfonitric acid mixture (HNO3 + H2SO4). Indicate the majority if necessary.arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning


