
Chemistry
3rd Edition
ISBN: 9780073402734
Author: Julia Burdge
Publisher: MCGRAW-HILL HIGHER EDUCATION
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 2.8, Problem 1PPC
Practice Problem CONCEPTUALIZE
CONCEPTUALIZE
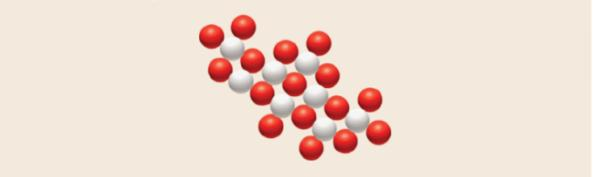
The diagram represents a small sample of an ionic compound where red spheres represent nitrate ions and grey spheres represent iron ions. Deduce the correct formula and name of the compound.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
EFFICIENTS
SAMPLE READINGS
CONCENTRATIONS
Pigiadient)
TOMATO SAUCE (REGULAR)
TOMATO (REDUCED SALT)
TOMATO SAUCE (REGULAR)
TOMATO (REDUCED SALT)
58
6.274
3.898
301.7
151.2
14150
5.277
3.865
348.9
254.8
B
5.136
3.639
193.7
85.9
605
4.655
3.041
308.6
199.6
05
5.135
3.664
339.5
241.4
0139
4.676
3.662
160.6
87.6
90148
5.086
3.677
337.7
242.5
0092
6.348
3.775
464.7
186.4
PART3
5.081
3.908
223.5
155.8
5.558
3.861
370.5
257.1
4.922
3.66
326.6
242.9
4.752
3.641
327.5
253.3
50
5.018
3.815
336.1
256.0
84
4.959
3.605
317.9
216.6
38
4.96
3.652
203.8
108.7
$3
5.052
3.664
329.8
239.0
17
5.043
3.767
221.9
149.7
052
5.058
3.614
331.7
236.4
5.051
4.005
211.7
152.1
62
5.047
3.637
309.6
222.7
5.298
3.977
223.4
148.7
5.38
4.24
353.7
278.2
5
5.033
4.044
334.6
268.7
995
4.706
3.621
305.6
234.4
04
4.816
3.728
340.0
262.7
16
4.828
4.496
304.3
283.2
0.011
4.993
3.865
244.7
143.6
AVERAGE
STDEV
COUNT
95% CI Confidence Interval (mmol/L)
[Na+] (mg/100 mL)
95% Na+ Confidence Interval (mg/100 mL)
If we have two compounds: acetone (CH₃COCH₃) and acetic acid (CH₃COOH), applying heat to them produces an aldol condensation of the two compounds. If this is correct, draw the formula for the final product.
If we have two compounds: acetone (CH3COCH3)
and acetic acid (CH3COOH); if we apply heat (A),
what product(s) are obtained?
Chapter 2 Solutions
Chemistry
Ch. 2.1 - Practice Problem ATTEMPT
In each case, calculate...Ch. 2.1 - Practice Problem BUILD
(a) Two of the simplest...Ch. 2.1 - Practice Problem CONCEPTUALIZE
Which of the...Ch. 2.1 - For the two compounds pictured, evaluate the...Ch. 2.1 - Prob. 2CPCh. 2.2 - Practice ProblemATTEMPT How many protons,...Ch. 2.2 - Practice ProblemBUILD Give the correct symbols to...Ch. 2.2 - Prob. 1PPCCh. 2.3 - 2.3.1 How many neutrons are there in an atom of
Ch. 2.3 - Prob. 2CP
Ch. 2.3 - Practice ProblemATTEMPT The atomic masses of the...Ch. 2.3 - Practice ProblemBUILD The average atomic mass of...Ch. 2.3 - Practice Problem CONCEPTUALIZE
The following...Ch. 2.4 - Which of the following series of elemental symbols...Ch. 2.4 - 2.4.2 Which of the following elements would you...Ch. 2.4 - Practice ProblemATTEMPT Chloroform was used as an...Ch. 2.4 - Practice ProblemBUILD Write the molecular formula...Ch. 2.4 - Prob. 1PPCCh. 2.5 - Boron has two naturally occurring isotopes, which...Ch. 2.5 - 2.5.2 The two naturally occurring isotopes of...Ch. 2.5 - Practice Problem ATTEMPT
Name the following...Ch. 2.5 - Practice ProblemBUILD Name the following binary...Ch. 2.5 - Practice Problem CONCEPTUALIZE
Name the binary...Ch. 2.6 - Practice ProblemATTEMPT Give the molecular formula...Ch. 2.6 - Practice ProblemBUILD Give the molecular formula...Ch. 2.6 - Prob. 1PPCCh. 2.6 - Prob. 1CPCh. 2.6 - 2.7.2 What is the name of the compound...Ch. 2.6 - What is the correct formula for the compound...Ch. 2.6 - 2.7.4 What is the empirical formula of the...Ch. 2.7 - Prob. 1CPCh. 2.7 - Prob. 2CPCh. 2.7 - Prob. 3CPCh. 2.7 - 2.6.4 What is the formula of nickel(II) nitrate...Ch. 2.7 - Prob. 5CPCh. 2.7 - Prob. 6CPCh. 2.7 - Practice Problem ATTEMPT
Write empirical formulas...Ch. 2.7 - Practice ProblemBUILD For which of the following...Ch. 2.7 - Practice ProblemCONCEPTUALIZE Which of the...Ch. 2.8 - Practice Problem ATTEMPT
Name the following ionic...Ch. 2.8 - Practice Problem BUILD
Name the following ionic...Ch. 2.8 - Practice ProblemCONCEPTUALIZE The diagram...Ch. 2.9 - Practice Problem ATTEMPT
Deduce the formulas of...Ch. 2.9 - Practice ProblemBUILD Deduce the formulas of the...Ch. 2.9 - Practice Problem CONCEPTUALIZE
The diagram...Ch. 2.10 - Name the following species:...Ch. 2.10 - Name the following species:...Ch. 2.10 - Prob. 1PPCCh. 2.11 - Practice ProblemATTEMPT Determine the formula of...Ch. 2.11 - Practice ProblemBUILD Determine the formula of...Ch. 2.11 - Practice ProblemCONCEPTUALIZE Referring to the...Ch. 2 - Prob. 1KSPCh. 2 - Prob. 2KSPCh. 2 - Prob. 3KSPCh. 2 - What is the correct formula for phosphorus...Ch. 2 - What are the hypotheses on which Dalton's atomic...Ch. 2 - State the laws of definite proportions and...Ch. 2 - Prob. 3QPCh. 2 - Prob. 4QPCh. 2 - 2.5 Sulfur reacts with fluorine to produce three...Ch. 2 - 2.6 Both and contain only iron and oxygen. The...Ch. 2 - For the two compounds pictured, evaluate the...Ch. 2 - 2.8 For the two compounds pictured, evaluate the...Ch. 2 - Prob. 9QPCh. 2 - Prob. 10QPCh. 2 - Prob. 11QPCh. 2 - Describe the contributions of the following...Ch. 2 - 2.13 Describe the experimental basis for believing...Ch. 2 - The diameter of a neutral helium atom is about 1 ×...Ch. 2 - Prob. 15QPCh. 2 - Prob. 16QPCh. 2 - Prob. 17QPCh. 2 - Prob. 18QPCh. 2 - Prob. 19QPCh. 2 - 2.20 What is the mass number of an iron atom that...Ch. 2 - Prob. 21QPCh. 2 - 2.22 For each of the following species, determine...Ch. 2 - 2.23 Indicate the number of protons, neutrons, and...Ch. 2 - Prob. 24QPCh. 2 - Prob. 25QPCh. 2 - Prob. 26QPCh. 2 - Prob. 27QPCh. 2 - Prob. 28QPCh. 2 - What is the periodic table, and what is its...Ch. 2 - 2.30 State two differences between a metal and a...Ch. 2 - Prob. 31QPCh. 2 - Give two examples of each of the following: (a)...Ch. 2 - Prob. 33QPCh. 2 - Prob. 34QPCh. 2 - Prob. 35QPCh. 2 - Prob. 36QPCh. 2 - Prob. 37QPCh. 2 - Prob. 38QPCh. 2 - Prob. 39QPCh. 2 - Prob. 40QPCh. 2 - Prob. 41QPCh. 2 - Prob. 42QPCh. 2 - Prob. 43QPCh. 2 - The atomic masses of 1735Cl(75.53percent) and...Ch. 2 - The atomic masses of 204 Pb ( 1 .4 percent ) . 206...Ch. 2 - Prob. 46QPCh. 2 - Prob. 47QPCh. 2 - 2.48 What is the mass in grams of 13.2 amu?
Ch. 2 - Prob. 49QPCh. 2 - Prob. 71QPCh. 2 - Prob. 72QPCh. 2 - Prob. 73QPCh. 2 - Prob. 74QPCh. 2 - Prob. 75QPCh. 2 - Prob. 76QPCh. 2 - Prob. 77QPCh. 2 - Prob. 78QPCh. 2 - Prob. 79QPCh. 2 - Prob. 80QPCh. 2 - 2.61 Name the following compounds:
Ch. 2 - Prob. 82QPCh. 2 - Prob. 83QPCh. 2 - Prob. 84QPCh. 2 - Prob. 85QPCh. 2 - Prob. 86QPCh. 2 - Prob. 50QPCh. 2 - Prob. 51QPCh. 2 - Prob. 52QPCh. 2 - Prob. 53QPCh. 2 - Prob. 54QPCh. 2 - Prob. 55QPCh. 2 - Prob. 56QPCh. 2 - Prob. 57QPCh. 2 - Prob. 58QPCh. 2 - Prob. 59QPCh. 2 - Prob. 60QPCh. 2 - Prob. 61QPCh. 2 - Prob. 62QPCh. 2 - Prob. 63QPCh. 2 - Prob. 64QPCh. 2 - Prob. 65QPCh. 2 - Prob. 66QPCh. 2 - Prob. 67QPCh. 2 - Prob. 68QPCh. 2 - Prob. 69QPCh. 2 - Prob. 70QPCh. 2 - Prob. 87APCh. 2 - Prob. 88APCh. 2 - Prob. 89APCh. 2 - Prob. 90APCh. 2 - Prob. 91APCh. 2 - Prob. 92APCh. 2 - 2.93 What is wrong with or ambiguous about the...Ch. 2 - Prob. 94APCh. 2 - Prob. 95APCh. 2 - Prob. 96APCh. 2 - Prob. 97APCh. 2 - Prob. 98APCh. 2 - Prob. 99APCh. 2 - Prob. 100APCh. 2 - Prob. 101APCh. 2 - Prob. 102APCh. 2 - Prob. 103APCh. 2 - Determine the molecular and empirical formulas of...Ch. 2 - Prob. 105APCh. 2 - Prob. 106APCh. 2 - The Group 1B metals . Cu, Ag, and Au, are called...Ch. 2 - Prob. 108APCh. 2 - Prob. 109APCh. 2 - Prob. 110APCh. 2 - Two elements form a compound that can be...Ch. 2 - Which of the diagrams can be used to illustrate...Ch. 2 - Prob. 113APCh. 2 - Prob. 114APCh. 2 - Prob. 115APCh. 2 - 2.116 Show the locations of (a) alkali metals, (b)...Ch. 2 - Prob. 117APCh. 2 - Prob. 118APCh. 2 - Prob. 119APCh. 2 - 2.120 (a) Describe Rutherford’s experiment and how...Ch. 2 - Prob. 121APCh. 2 - Prob. 122APCh. 2 - Prob. 123APCh. 2 - A cube made of platinum (Pt) has an edge length of...Ch. 2 - Prob. 125APCh. 2 - Prob. 126APCh. 2 - Prob. 1SEPPCh. 2 - Prob. 2SEPPCh. 2 - Prob. 3SEPPCh. 2 - Prob. 4SEPP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- QUESTION: Fill out the answers to the empty green boxes attached in the image. *Ensure you all incorporate all 27 values (per column)*arrow_forwardYou need to make a buffer by dissolving benzoic acid and sodium benzoate in water. What is the mass of benzoic acid that you would weigh out, in mg, to create 50 mL of a buffer at pH = 4.7 that will change pH no more than 0.10 units with the addition of 0.001 moles of acid or base? Enter just the answer without the units (mg) - just the number will do!arrow_forwardDraw the formula for 3-isopropylcyclopentane-1-carbonyl chloride.arrow_forward
- QUESTION: Fill out the answers to the empty green boxes attached in the image. *Ensure you all incorporate all 27 values (per column)*arrow_forwardComplete the following reactions- hand written pleasearrow_forwardGive the organic product: O A O B Ос ○ D -NH–CH3 + CH3 CH3 NEN C ? A CH3 CH3 NH- CH3 B CH3 CH3 N=N- C CH3 CH3 N=NNH CH3 D CH3 N=N CH3 NHCH3 LNH CHOarrow_forward
- Finish the reaction- hand written pleasearrow_forwardGive the organic products: (benzyne) Br ? CH3 + K* :NH, liq NH3 HINT: Two products are formed. Each is a substituted aniline; they are isomers of each other. NH2 II I H₂N. CH3 CH3 III Select one: ○ A. I and II ○ B. I and III O C. I and IV O D. II and III O E. III and IV H₂N CH3 IV CH₂-NH2arrow_forwardPredict the major products of this organic reaction: HBr (1 equiv) cold ? Some important notes: • Draw the major product, or products, of this reaction in the drawing area below. • You can draw the products in any arrangement you like. • Pay careful attention to the reaction conditions, and only include the major products. • Be sure to use wedge and dash bonds when necessary, for example to distinguish between major products that are enantiomers. • Note that there is only 1 equivalent of HBr reactant, so you need not consider the case of multiple additions. Erase something Explanation Check 2025 McGraw Hill LLC. All Rights Reserved. Terarrow_forward
- Q14. Fill this chart: (please refer to ppt notes/browser to answer these questions) What alcohol is also called wood alcohol? What is the common name of ethanol? Draw the structure of phenol and thiophene? Are bigger chain alcohol like heptanol and octanol are soluble or insoluble in water and explain it ? Are ethers soluble or insoluble in water? What suffix and prefix are used for alcohol while naming alcohol and ether? What the process called when we add water to any alkene to make alcohol? Q16. Draw the diagram of following aromatic compound (practice from previous module) Aniline Phenol Benzoic acid Methyl benzoate Q17. a. Write the oxidation reactions for the 2 propanol. b. Write the oxidation reaction of the ethanol.arrow_forwardQuestion 11 of 18 (1 point) Question Attempt: 3 of How many signals do you expect in the 'H NMR spectrum for this molecule? Br Br Write the answer below. Also, in each of the drawing areas below is a copy of the molecule, with Hs shown. In each copy, one of the H atoms is colored red. Highlight in red all other H atoms that would contribute to the same signal as the H already highlighted red. Note for advanced students: In this question, any multiplet is counted as one signal. Number of signals in the 'H NMR spectrum. 1 For the molecule in the top drawing area, highlight in red any other H atoms that will contribute to the same signal as the H atom already highlighted red. If no other H atoms will contribute, check the box at right. No additional Hs to color in top molecule Check For the molecule in the bottom drawing area, highlight in red any other H atoms that will contribute to the same signal as the H atom already highlighted red. If no other H atoms will contribute, check the box…arrow_forwardOrganic Chemistry Esterification reactions 1. Write the steps to prepare ester. 2. Write complete reaction of ethanol and acetic acid to make ester. 3. What does ester smell like? What are the uses of ester. 4. What the role of sulfuric acid in the esterification reactionarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning
Periodic Properties of Elements | Chemistry | IIT-JEE | NEET | CBSE | Misostudy; Author: Misostudy;https://www.youtube.com/watch?v=L26rRWz4_AI;License: Standard YouTube License, CC-BY
Periodic Trends: Electronegativity, Ionization Energy, Atomic Radius - TUTOR HOTLINE; Author: Melissa Maribel;https://www.youtube.com/watch?v=0h8q1GIQ-H4;License: Standard YouTube License, CC-BY