
ORGANIC CHEMISTRY
6th Edition
ISBN: 9781266633973
Author: SMITH
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 27, Problem 70P
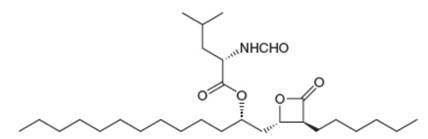
The anti-obesity drug orlistat works by irreversibly inhibiting pancreatic lipase, an enzyme responsible for the hydrolysis of triacylglycerols in the intestines, so they are excreted without
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
The approximation of calculating the partition function by integration instead of the summation of all the energy terms can only be done if the separation of the energy levels is much smaller than the product kT. Explain why.
Explain the meaning of: the electron partition function is equal to the degeneracy of the ground state.
28. For each of the following species, add charges wherever required to give
a complete, correct Lewis structure. All bonds and nonbonded valence
electrons are shown.
a.
b.
H
H
H
H
H
:0-C-H
H
H
H-C-H
C.
H
H
d. H-N-0:
e.
H
H-O
H-O
H
B=0
f. H—Ö—Ñ—Ö—H
Norton Private B
Chapter 27 Solutions
ORGANIC CHEMISTRY
Ch. 27.1 - Prob. 1PCh. 27.1 - Problem 29.2
What form exists at the isoelectric...Ch. 27.1 - Problem 29.3
Explain why the of the group of an...Ch. 27.1 - Prob. 4PCh. 27.2 - Problem 29.5
What -halo carbonyl compound is...Ch. 27.2 - Problem 29.6
The enolate derived from diethyl...Ch. 27.2 - Problem 29.7
What amino acid is formed when is...Ch. 27.2 - Problem 29.8
What aldehyde is needed to synthesize...Ch. 27.2 - Problem 29.9
Draw the products of each...Ch. 27.3 - Prob. 10P
Ch. 27.3 - Prob. 11PCh. 27.3 - Prob. 12PCh. 27.4 - Problem 29.13
What alkene is needed to synthesize...Ch. 27.5 - Problem 29.14
Draw the structure of each peptide....Ch. 27.5 - Problem 29.15
Name each peptide using both the...Ch. 27 - Draw the product formed when the following amino...Ch. 27 - With reference to the following peptide: a...Ch. 27 - Prob. 31PCh. 27 - Histidine is classified as a basic amino acid...Ch. 27 - Tryptophan is not classified as a basic amino acid...Ch. 27 - What is the structure of each amino acid at its...Ch. 27 - What is the predominant form of each of the...Ch. 27 - 29.37 What is the predominant form of each of the...Ch. 27 - a. Draw the structure of the tripeptide A–A–A, and...Ch. 27 - 29.39 Draw the organic products formed in each...Ch. 27 - 29.40 What alkyl halide is needed to synthesize...Ch. 27 - Prob. 50PCh. 27 - Draw the structure for each peptide: (a) Phe–Ala;...Ch. 27 - 29.52 For the tetrapeptide Asp–Arg–Val–Tyr:
a....Ch. 27 - Prob. 53PCh. 27 - Prob. 54PCh. 27 - 29.55 Draw the amino acids and peptide fragments...Ch. 27 - Prob. 56PCh. 27 - Prob. 57PCh. 27 - Prob. 58PCh. 27 - 29.59 An octapeptide contains the following amino...Ch. 27 - 29.60 Draw the organic products formed in each...Ch. 27 - 29.65 Draw the mechanism for the reaction that...Ch. 27 - 29.66 Which of the following amino acids are...Ch. 27 - 29.67 After the peptide chain of collagen has been...Ch. 27 - Prob. 68PCh. 27 - Prob. 69PCh. 27 - 29.70 The anti-obesity drug orlistat works by...Ch. 27 - Prob. 71P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- At 0oC and 1 atm, the viscosity of hydrogen (gas) is 8.55x10-5 P. Calculate the viscosity of a gas, if possible, consisting of deuterium. Assume that the molecular sizes are equal.arrow_forwardIndicate the correct option for the velocity distribution function of gas molecules:a) its velocity cannot be measured in any other way due to the small size of the gas moleculesb) it is only used to describe the velocity of particles if their density is very high.c) it describes the probability that a gas particle has a velocity in a given interval of velocitiesd) it describes other magnitudes, such as pressure, energy, etc., but not the velocity of the moleculesarrow_forwardIndicate the correct option for the velocity distribution function of gas molecules:a) its velocity cannot be measured in any other way due to the small size of the gas moleculesb) it is only used to describe the velocity of particles if their density is very high.c) it describes the probability that a gas particle has a velocity in a given interval of velocitiesd) it describes other magnitudes, such as pressure, energy, etc., but not the velocity of the moleculesarrow_forward
- The number of imaginary replicas of a system of N particlesA) can never become infiniteB) can become infiniteC) cannot be greater than Avogadro's numberD) is always greater than Avogadro's number.arrow_forwardElectronic contribution to the heat capacity at constant volume A) is always zero B) is zero, except for excited levels whose energy is comparable to KT C) equals 3/2 Nk D) equals Nk exp(BE)arrow_forwardPlease correct answer and don't used hand raitingarrow_forward
- Calculate the packing factor of CaTiO3. It has a perovskite structure. Data: ionic radii Co²+ = 0.106 nm, Ti4+ = 0.064 nm, O² = 0.132 nm; lattice constant is a = 2(rTi4+ + ro2-). Ca2+ 02- T14+ Consider the ions as rigid spheres. 1. 0.581 or 58.1% 2. -0.581 or -58.1 % 3. 0.254 or 25.4%arrow_forwardGeneral formula etherarrow_forwardPlease provide the retrosynthetic analysis and forward synthesis of the molecule on the left from the starting material on the right. Please include hand-drawn structures! will upvote! Please correct answer and don't used hand raitingarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div
World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

World of Chemistry
Chemistry
ISBN:9780618562763
Author:Steven S. Zumdahl
Publisher:Houghton Mifflin College Div
DIGESTER-35 | VITAMINS AND THEIR RELATED COENZYMES| GPAT | NIPER | PHARMACIST| DI; Author: GPAT DISCUSSION CENTER;https://www.youtube.com/watch?v=CGrdNYmho0s;License: Standard YouTube License, CC-BY